History and exam
Key diagnostic factors
common
presence of risk factors
Key risk factors include increased skin pigmentation, obesity, malabsorption syndromes, history of liver failure or chronic kidney disease, adults aged >50 years, history of tumour, and patients taking glucocorticoids, anti-epileptic medications, highly active antiretroviral therapy, rifampicin, or St John's wort.[2][68] Children and adults who live in cold climates, avoid all sun exposure, or always wear sun protection outdoors and do not take a vitamin D supplement are also at high risk, as are neonates whose sole source of nutrition is breast milk and who receive no vitamin D supplementation.[1][60]
bowing of the legs
Children with rickets will develop either an inward or an outward bowing of the femur and tibia once they begin to stand and walk.[Figure caption and citation for the preceding image starts]: Inward or outward bowing of the legs is a typical sign of classic ricketsCDC [Citation ends].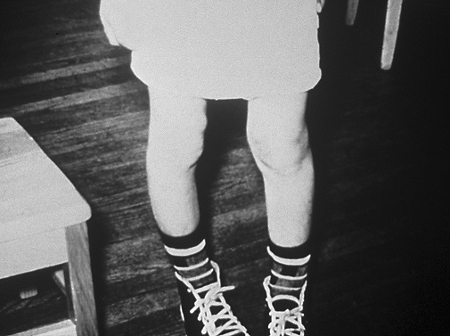
widening of the ends of the long bones
In children, vitamin D deficiency causes hypertrophy of the epiphyseal plates of the long bones, resulting in widening of the wrists. This can be verified with x-ray, which shows ragged poor mineralisation of the epiphyseal plates. [Figure caption and citation for the preceding image starts]: X-rays of a wrist from a child with vitamin D-deficiency rickets before (left panel) and after (right panel) treatment with vitamin DFrom the collection of M.F. Holick, PhD, MD; used with permission [Citation ends].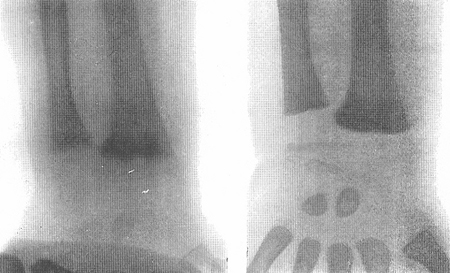
delayed tooth eruption and early dental caries
Occurs in children with rickets.
chest deformity
Occurs in children with rickets, and is due to muscle traction on the softened ribcage leading to pectus carinatum, thoracic asymmetry, and a widening of the thoracic base.
throbbing, aching bone discomfort and/or irritability
Bone discomfort is typical of prolonged or severe vitamin D deficiency in adults (osteomalacia). Patients often report bone pain when sitting or lying down and describe the pain as unrelenting and throbbing.
In infants or young children with rickets, bone pain is usually expressed as irritability.
head sweating
Can occur in children with severe vitamin D deficiency, due to an increase in neuromuscular activity.
localised or generalised bone tenderness
Periosteal bone discomfort consistent with the diagnosis of osteomalacia can be elicited by pressing with a fair amount of force on the sternum, radius, and anterior tibia using thumb or forefinger.
proximal muscle weakness
Adults with chronic vitamin D deficiency often have muscle weakness, especially of the proximal muscles in their legs. This is evident by watching the patient get up from a sitting position with difficulty.
uncommon
rachitic rosary
Refers to knob-like projections along the ribcage occurring in children with rickets.
frontal bossing
Can occur in a child with longstanding rickets; due to an increase in bone formation and flattening of the forehead.
waddling gait
Due to pain in the hips, and is a sign of osteomalacia.
Other diagnostic factors
common
faltering growth
Growth retardation is a common presentation of rickets, and is due to poor global mineralisation of the skeleton.
delayed achievement of motor milestones
Common presentation of rickets as a result of poor global mineralisation of the skeleton.
fatigue and malaise
Common symptoms in patients with osteomalacia.
uncommon
symptoms of hypocalcaemia
Include muscle cramps, muscle weakness, carpal pedal spasm, numbness, paraesthesias, tetany, and seizures.
Can occur as a result of inherited disorders of vitamin D metabolism or chronic untreated vitamin D deficiency. Carpal pedal spasms and generalised seizures can occur due to increased neuromuscular irritability.
Risk factors
strong
inadequate sunlight exposure
Sunlight is the major source of vitamin D for most humans.[2] Therefore, avoiding sun exposure, heavy sunscreen use, season, latitude, and time of day influence cutaneous vitamin D production.[1][58][70]
Living above or below a latitude of 35° either markedly reduces or completely eliminates any vitamin D production from sunlight exposure in the winter.[58]
Vitamin D photosynthesis depends on many factors including genetics, age, health, and personal and cultural behaviour.[70] Particularly susceptible groups include older people (especially if institutionalised) and women and children who are confined to the home, or who wear clothing that covers the entire body and face.[58]
increased skin pigmentation
age >50 years
Ageing does not affect vitamin D absorption from the diet, but it does decrease the ability of the skin to produce vitamin D.[58]
The increase in the blood level of vitamin D following ultraviolet B radiation exposure is at least three-fold higher in young adults aged 20-30 years, compared with older adults aged 62-80 years.[58]
inadequate dietary and supplemental vitamin D intake
There are high levels of inadequacy of vitamin D intake within populations around the globe, in part due to variability in availability of vitamin-D rich food sources and differences in the practice of food fortification between countries.[11] Most vitamin D-fortified foods contain 100 IU vitamin D/serving. Oily fish such as wild salmon have about 500 to 1000 IU/100 g. Most estimates do not include the contribution from vitamin D-containing dietary supplements.[11]
Infants receiving breast milk as their sole source of nutrition, without vitamin D supplementation, are most susceptible.[60]
malabsorption
Patients with coeliac disease, cystic fibrosis, Crohn's disease, Whipple's disease, or short bowel syndrome, or who have undergone gastric bypass surgery, are unable to absorb or poorly absorb vitamin D.[2][63]
Severe liver failure is associated with vitamin D deficiency.[64] When >90% of the liver fails, it is incapable of producing enough 25-hydroxyvitamin D.[1][3]
obesity
Body fat stores vitamin D. However, when BMI is >30 the fat sequesters vitamin D, thus increasing the risk for vitamin D deficiency.[66]
medication use
Includes the use of glucocorticoids, antiepileptic medication, highly active antiretroviral therapy, rifampicin, and St John's wort. These drugs activate the steroid and xenobiotic receptors, which results in activation of the enzymatic machinery destroying 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D.[68]
genetic mutations
Inherited disorders of vitamin D metabolism and 1,25-dihydroxyvitamin D recognition are rare.[5]
Vitamin D-dependent rickets type 1 is caused by a mutations of genes that generate 25-hydroxyvitamin-1-hydroxylase or 25-hydroxylase.[6]
Vitamin D-dependent rickets type 2 is caused by a mutation of the vitamin D receptor (VDR) gene.[6]
X-linked hypophosphataemic rickets and autosomal-dominant hypophosphataemic rickets are caused by the enhanced production or decreased metabolism of fibroblast growth factor 23. This results in hyperphosphaturia, severe hypophosphataemia, and reduction in the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D.[8]
tumour
Whether benign or malignant, can produce fibroblast growth factor 23, causing hypophosphataemia and low levels of serum 1,25-dihydroxyvitamin D, with consequent oncogenic osteomalacia and pronounced biochemical and skeletal manifestations.[4]
chronic kidney disease
The kidneys activate vitamin D in order to regulate calcium metabolism. Chronic kidney disease (CKD) results in retention of phosphate causing an increase in the production of fibroblast growth factor 23 (FGF-23) by osteocytes. The FGF-23 inhibits the production of 1,25-dihydroxyvitamin D and initially results in secondary hyperparathyroidism.[9] Patients with later stage CKD with an eGFR <45 mL/minute/1.73 m² (category stage G3b-G5 ) are unable to produce enough 1,25-dihydroxyvitamin D, leading to the development of CKD-metabolic bone disease.[69] All patients with CKD need to maintain their 25-hydroxyvitamin D level at >75 nanomoles/L (>30 nanograms/mL) to maximise their bone health.[1][10]
granulomatous disorders
Patients with granuloma-forming diseases, including sarcoidosis and tuberculosis, are at risk for vitamin D deficiency due to its enhanced destruction. Macrophages in the granuloma can cause increased metabolism of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, resulting in increased expression of the enzyme 24-hydroxylase, which in turn increases the destruction of both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, resulting in vitamin D deficiency.[2][3]
primary hyperparathyroidism
High PTH levels can cause increased metabolism of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, resulting in increased expression of the enzyme 24-hydroxylase, which in turn increases the destruction of both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, resulting in vitamin D deficiency.[2][3]
Use of this content is subject to our disclaimer