Deep vein thrombosis (DVT)
Acute-onset, asymmetrical, tender leg oedema (with associated swelling and redness) may be a sign of new DVT, which can be a threat to life if it embolises to the lung.[17]Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016 Dec 17;388(10063):3060-73.
http://www.ncbi.nlm.nih.gov/pubmed/27375038?tool=bestpractice.com
Patients may also report localized pain over the deep venous system or dilated superficial veins over the foot and leg.
[Figure caption and citation for the preceding image starts]: Deep vein thrombosis (DVT) of the right leg with associated swelling and rednessFrom the collection of James Heilman, MD. [Citation ends].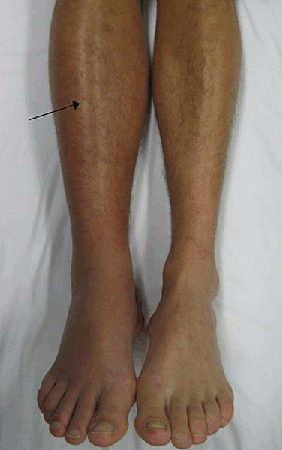
Massive venous thrombosis can be associated with a limb-threatening syndrome referred to as phlegmasia cerulea dolens, in which the venous congestion leads to such a degree of interstitial oedema that compartment syndrome, arterial compromise, and gangrene can occur.[18]Bhatt S, Wehbe C, Dogra VS. Phlegmasia cerulea dolens. J Clin Utrasound. 2007 Sep;35(7):401-4.
http://www.ncbi.nlm.nih.gov/pubmed/17354247?tool=bestpractice.com
For patients with a high pre-test probability of DVT, determined by Wells clinical prediction rules, duplex ultrasound is the first-line test in most instances, particularly if a thrombosis in the popliteal, superficial femoral, or common femoral veins is suspected.
[
Modified Wells score for deep vein thrombosis (DVT)
Opens in new window
]
If the pre-test probability is low, a negative D-dimer test allows ultrasound to be safely excluded.[17]Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016 Dec 17;388(10063):3060-73.
http://www.ncbi.nlm.nih.gov/pubmed/27375038?tool=bestpractice.com
[19]Kakkos SK, Gohel M, Baekgaard N, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur J Vasc Endovasc Surg. 2021 Jan;61(1):9-82.
https://www.ejves.com/article/S1078-5884(20)30868-6/fulltext#secsectitle0630
http://www.ncbi.nlm.nih.gov/pubmed/33334670?tool=bestpractice.com
In the absence of contraindications, therapeutic anticoagulation should be initiated immediately on diagnosis.[17]Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016 Dec 17;388(10063):3060-73.
http://www.ncbi.nlm.nih.gov/pubmed/27375038?tool=bestpractice.com
[19]Kakkos SK, Gohel M, Baekgaard N, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur J Vasc Endovasc Surg. 2021 Jan;61(1):9-82.
https://www.ejves.com/article/S1078-5884(20)30868-6/fulltext#secsectitle0630
http://www.ncbi.nlm.nih.gov/pubmed/33334670?tool=bestpractice.com
Further treatment, such as thrombolysis, thrombectomy, and/or fasciotomy, may be necessary for massive thrombosis.[19]Kakkos SK, Gohel M, Baekgaard N, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur J Vasc Endovasc Surg. 2021 Jan;61(1):9-82.
https://www.ejves.com/article/S1078-5884(20)30868-6/fulltext#secsectitle0630
http://www.ncbi.nlm.nih.gov/pubmed/33334670?tool=bestpractice.com
[20]Singh AD, Makkar N, Ray A, et al. Phlegmasia cerulea dolens presenting with acute compartment syndrome and pulmonary embolism. BMJ Case Rep. 2018 Jun 13;2018:bcr2018224879.
https://casereports.bmj.com/content/2018/bcr-2018-224879.long
Heart failure
Acute-onset heart failure, which may present with dyspnoea and lower-extremity oedema (usually bilateral), requires urgent evaluation to establish the underlying aetiology of the cardiac dysfunction.[21]Hunter BR, Martindale J, Abdel-Hafez O, et al. Approach to acute heart failure in the emergency department. Prog Cardiovasc Dis. 2017 Sep - Oct;60(2):178-86.
https://www.doi.org/10.1016/j.pcad.2017.08.008
http://www.ncbi.nlm.nih.gov/pubmed/28865801?tool=bestpractice.com
Emergency diagnoses such as myocardial ischaemia, pericardial effusion with cardiac tamponade, constrictive pericarditis, valvular disease, or cardiomyopathy should be considered when new symptoms of heart failure develop rapidly.[22]Allen LA, O'Connor CM. Management of acute decompensated heart failure. CMAJ. 2007 Mar 13;176(6):797-805.
https://www.cmaj.ca/content/176/6/797.full
http://www.ncbi.nlm.nih.gov/pubmed/17353535?tool=bestpractice.com
Complaints of chest pain, shortness of breath, orthopnoea, or paroxysmal nocturnal dyspnoea may provide a clue that the peripheral oedema arises from an acute cardiac cause.
Evidence of pulmonary oedema, elevated jugular venous pressure, heart murmurs, pericardial friction rubs, or pulsus paradoxus further support a cardiac aetiology of the oedema. ECG, chest x-ray, cardiac troponins, B-type natriuretic peptide/N-terminal pro-B-type natriuretic peptide, and echocardiography would be appropriate to evaluate for suspected acute cardiac disease when peripheral oedema is a component of the presenting syndrome.[23]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[24]National Institute for Health and Care Excellence. Heart valve disease presenting in adults: investigation and management. Nov 2021 [internet publication].
https://www.nice.org.uk/guidance/ng208
Exacerbation of pre-existing chronic heart failure is noticed by the patient as fluid retention and increased lower-extremity oedema.[22]Allen LA, O'Connor CM. Management of acute decompensated heart failure. CMAJ. 2007 Mar 13;176(6):797-805.
https://www.cmaj.ca/content/176/6/797.full
http://www.ncbi.nlm.nih.gov/pubmed/17353535?tool=bestpractice.com
A cause for the decompensation should be sought. Worsening of symptoms of oedema from known heart failure requires treatment to regain euvolaemia, usually loop diuretics, and institution of other measures to improve and preserve cardiac function.[22]Allen LA, O'Connor CM. Management of acute decompensated heart failure. CMAJ. 2007 Mar 13;176(6):797-805.
https://www.cmaj.ca/content/176/6/797.full
http://www.ncbi.nlm.nih.gov/pubmed/17353535?tool=bestpractice.com
[25]Bromage D, Mayhew J, Sado D. Managing heart failure related peripheral oedema in primary care. BMJ. 2020 Jun 22;369:m2099.
https://www.doi.org/10.1136/bmj.m2099
http://www.ncbi.nlm.nih.gov/pubmed/32571780?tool=bestpractice.com
Pericardial effusions with tamponade require pericardiocentesis.[26]Hoit BD. Pericardial disease and pericardial tamponade. Crit Care Med. 2007 Aug;35(8 suppl):S355-64.
http://www.ncbi.nlm.nih.gov/pubmed/17667460?tool=bestpractice.com
New-onset renal disease
Frequently, the presenting symptom of nephrotic syndrome is peripheral oedema, which starts in the legs and may become severe involving the entire body (anasarca), and peri-orbital oedema.[27]Politano SA, Colbert GB, Hamiduzzaman N. Nephrotic syndrome. Prim Care. 2020 Dec;47(4):597-613.
http://www.ncbi.nlm.nih.gov/pubmed/33121631?tool=bestpractice.com
Patients may also report frothy urine. Initial investigations should include testing for proteinuria, serum creatinine and serum albumin. Severe protein-wasting renal disease or acute renal failure with volume overload requires early nephrology evaluation.
Myxoedema coma
Peripheral oedema is one of the features seen with profound hypothyroidism, an endocrinological emergency.[28]Li S, Alsaiqali M, Narayanaswamy M, et al. The vicious cycle of hypothyroidism and severe proteinuria: a case report. Cureus. 2022 Sep;14(9):e28674.
https://pmc.ncbi.nlm.nih.gov/articles/PMC9526517
http://www.ncbi.nlm.nih.gov/pubmed/36199658?tool=bestpractice.com
In this setting, oedema would not occur in isolation but rather as part of a multi-system disorder. Other signs or symptoms may include mental status changes, lethargy, constipation, weight gain, coarse hair, dry skin, and cold intolerance.[29]Elkattawy S, Dhanoa P, Kotys J, et al. Myxedema coma: case report and literature review. Cureus. 2021 May 27;13(5):e15277.
https://pmc.ncbi.nlm.nih.gov/articles/PMC8235691
http://www.ncbi.nlm.nih.gov/pubmed/34194879?tool=bestpractice.com
Treatment requires intravenous thyroid hormone replacement.
Acute hepatic venous obstruction
Venous obstruction at the level of the liver (including Budd-Chiari syndrome and hepatic veno-occlusive disease) can cause peripheral oedema, typically in association with abdominal pain, hepatomegaly, and ascites.[30]Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: three similar syndromes. World J Gastroenterol. 2007 Apr 7;13(13):1912-27.
https://www.wjgnet.com/1007-9327/full/v13/i13/1912.htm
http://www.ncbi.nlm.nih.gov/pubmed/17461490?tool=bestpractice.com
While obstruction can be caused by a variety of pathological processes, thrombus formation would be associated with rapid onset of symptoms. The combination of peripheral oedema, hepatomegaly, ascites, and abdominal pain, along with elevated transaminases and possibly jaundice, should prompt evaluation of the blood flow through the liver with imaging such as duplex ultrasound. Computed tomography or magnetic resonance imaging can also be used. A similar picture of hepatic congestion can be seen with cardiac disease, and this should be maintained in the differential diagnosis for this presentation.
Compartment syndrome
Compartment syndrome can rapidly threaten the viability of a limb, and should be investigated and managed aggressively when suspected. Typical clinical scenarios include trauma to the extremity due to fractures, soft-tissue injury, vascular compromise, and burn injuries.
Features that might alert the clinician to this diagnosis include unilateral oedema, severe pain in the extremity especially on passive stretch of the muscle, a compartment tense to palpation, muscle weakness, and hypoaesthesia. Where applicable, casts or occlusive dressings should be removed and any padding or circumferential dressings should be released immediately.
Measurement of compartment pressure is indicated whenever the diagnosis is uncertain in a patient at risk. The perfusion pressure of a compartment (the compartment delta pressure) is defined as the difference between the diastolic BP and the intra-compartmental pressure (i.e., diastolic BP minus compartment pressure). A delta pressure of ≤30 mmHg is considered a strong indicator for fasciotomy.[31]Olson SA, Glasgow RR. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005 Nov;13(7):436-44.
https://www.doi.org/10.5435/00124635-200511000-00003
http://www.ncbi.nlm.nih.gov/pubmed/16272268?tool=bestpractice.com
[32]Shadgan B, Menon M, O'Brien PJ, et al. Diagnostic techniques in acute compartment syndrome of the leg. J Orthop Trauma. 2008 Sep;22(8):581-7.
http://www.ncbi.nlm.nih.gov/pubmed/18758292?tool=bestpractice.com
[33]McMillan TE, Gardner WT, Schmidt AH, et al. Diagnosing acute compartment syndrome-where have we got to? Int Orthop. 2019 Nov;43(11):2429-35.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6848051
http://www.ncbi.nlm.nih.gov/pubmed/31468110?tool=bestpractice.com
However, care should be taken when using this criterion in patients who are receiving vasodilatory medications and whose diastolic BP is low.
Sepsis
Peripheral oedema itself is not a typical presenting symptom for sepsis, unless the underlying cause is a focal infection in the limb.
In patients with sepsis, oedema may occur several days after the administration of large volume fluid resuscitation. Nevertheless, it is important to consider the possibility of sepsis in any patient with oedema who presents with possible signs of infection.[34]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
Sepsis is a spectrum of disease, where there is a systemic and dysregulated host response to an infection.[35]Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
https://www.doi.org/10.1001/jama.2016.0287
http://www.ncbi.nlm.nih.gov/pubmed/26903338?tool=bestpractice.com
Presentation ranges from subtle, non-specific symptoms (e.g., feeling unwell with a normal temperature, malaise, lethargy, nausea, or vomiting) to severe symptoms with evidence of multi-organ dysfunction and septic shock. Patients may have signs of tachycardia, tachypnoea, hypotension, fever or hypothermia, poor capillary refill, mottled or ashen skin, cyanosis, newly altered mental state or reduced urine output.[34]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
Sepsis and septic shock are medical emergencies.
Risk factors for sepsis include:[34]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
Age under 1 year
Age over 75 years
Frailty, impaired immunity (due to illness or drugs)
Recent surgery or other invasive procedures
Any breach of skin integrity (e.g., cuts, burns)
Intravenous drug misuse
Indwelling lines or catheters, and
Pregnancy or recent pregnancy
Early recognition of sepsis is essential because early treatment improves outcomes.[34]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
[36]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx
[Evidence C]0334601c-d597-4bce-9088-87f7e68c7a81guidelineCWhat are the effects of early versus late initiation of empiric antimicrobial treatment in adults with or at risk of developing sepsis or severe sepsis?[34]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
[Evidence C]8251b90b-b4ca-419f-ba77-1b666cb5eda1guidelineCWhat are the effects of early versus late initiation of empiric antimicrobial treatment in children with or at risk of developing sepsis or severe sepsis?[34]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
However, detection can be challenging because the clinical presentation of sepsis can be subtle and non-specific. A low threshold for suspecting sepsis is therefore important. The key to early recognition is the systematic identification of any patient who has signs or symptoms suggestive of infection and is at risk of deterioration due to organ dysfunction. Several risk stratification approaches have been proposed. All rely on a structured clinical assessment and recording of the patient’s vital signs.[34]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
[37]Royal College of Physicians. National Early Warning Score (NEWS) 2. December 2017 [internet publication].
http://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2
[38]American College of Emergency Physicians (ACEP) Expert Panel on Sepsis. DART: an evidence-driven tool to guide the early recognition and treatment of sepsis and septic shock [internet publication].
https://poctools.acep.org/POCTool/Sepsis(DART)/276ed0a9-f24d-45f1-8d0c-e908a2758e5a
[39]Academy of Medical Royal Colleges. Statement on the initial antimicrobial treatment of sepsis. May 2022 [internet publication].
https://www.aomrc.org.uk/reports-guidance/statement-on-the-initial-antimicrobial-treatment-of-sepsis
[40]Schlapbach LJ, Watson RS, Sorce LR, et al. International consensus criteria for pediatric sepsis and septic shock. JAMA. 2024 Feb 27;331(8):665-74.
https://jamanetwork.com/journals/jama/fullarticle/2814297
http://www.ncbi.nlm.nih.gov/pubmed/38245889?tool=bestpractice.com
It is important to check local guidance for information on which approach your institution recommends. The timeline of ensuing investigations and treatment should be guided by this early assessment.[39]Academy of Medical Royal Colleges. Statement on the initial antimicrobial treatment of sepsis. May 2022 [internet publication].
https://www.aomrc.org.uk/reports-guidance/statement-on-the-initial-antimicrobial-treatment-of-sepsis
Treatment guidelines have been produced by the Surviving Sepsis Campaign and remain the most widely accepted standards.[36]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx
[41]Society of Critical Care Medicine. Surviving Sepsis Campaign. Hour-1 Bundle. 2019 [internet publication].
https://www.sccm.org/getattachment/SurvivingSepsisCampaign/Guidelines/Adult-Patients/Surviving-Sepsis-Campaign-Hour-1-Bundle.pdf?lang=en-US
Recommended treatment of patients with suspected sepsis is:
Measure lactate level, and re-measure lactate if initial lactate is elevated (>2 mmol/L [>18 mg/dL]).
Obtain blood cultures before administering antibiotics.
Administer broad-spectrum antibiotics (with methicillin-resistant Staphylococcus aureus [MRSA] coverage if there is high risk of MRSA) for adults with possible septic shock or a high likelihood for sepsis.
For adults with sepsis or septic shock at high risk of fungal infection, empiric antifungal therapy should be administered.
Begin rapid administration of crystalloid fluids for hypotension or lactate level ≥4 mmol/L (≥36 mg/dL). Consult local protocols.
Administer vasopressors peripherally if hypotensive during or after fluid resuscitation to maintain MAP ≥65 mmHg, rather than delaying initiation until central venous access is secured. Noradrenaline (norepinephrine) is the vasopressor of choice.
For adults with sepsis-induced hypoxaemic respiratory failure, high flow nasal oxygen should be given.
Ideally these interventions should all begin in the first hour after sepsis recognition.[41]Society of Critical Care Medicine. Surviving Sepsis Campaign. Hour-1 Bundle. 2019 [internet publication].
https://www.sccm.org/getattachment/SurvivingSepsisCampaign/Guidelines/Adult-Patients/Surviving-Sepsis-Campaign-Hour-1-Bundle.pdf?lang=en-US
For adults with possible sepsis without shock, if concern for infection persists, antibiotics should be given within three hours from the time when sepsis was first recognised.[36]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx
For adults with a low likelihood of infection and without shock, antibiotics can be deferred while continuing to closely monitor the patient.[36]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx
See Sepsis in adults and Sepsis in children.
