Approach
The presenting symptoms and signs of ventricular tachycardia (VT) and supraventricular tachycardias (SVTs) share considerable overlap. With the exception of signs of AV dissociation (e.g., cannon A waves) that are essentially diagnostic of VT, few clinical findings can definitively identify the source of a wide complex tachycardia.
History
A history of acute or prior myocardial infarction, or depressed left ventricular systolic dysfunction, strongly favours a diagnosis of VT, but the converse is not necessarily true. Most symptoms associated with ventricular tachycardia are non-specific in that they may also be present in patients with other conditions, including SVT. For example, palpitations, dyspnoea, chest discomfort, and nausea/diaphoresis are common in patients with VT, but not pathognomonic. In addition, hypotension does not help to identify the origin of their arrhythmia; many instances of well-tolerated VT and of poorly tolerated SVT have been described. The patient may have a history of syncope or presyncope.
A family history of sudden death, especially if at an early age, should alert the physician to seek potential arrhythmogenic causes. Particularly if sudden death did not occur in the setting of underlying coronary artery disease, the physician must pay even more careful attention to screen for primary electrical abnormalities such as long QT syndrome and Brugada syndrome. In addition, imaging modalities including echocardiography and cardiac magnetic resonance imaging (MRI) should be considered, to evaluate for evidence of conditions such as hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and left ventricular non-compaction.
Patients at high risk for VT who should be considered for prophylactic implantable cardioverter defibrillator (ICD) include those with:
Ischaemic or non-ischaemic cardiomyopathy (left ventricular ejection fraction [LVEF] ≤35%) and mild-to-moderate congestive heart failure symptoms (New York Heart Association class II or III symptoms). Those with severe heart failure (class IV) are at high risk as well, but have progressive heart failure as a competing mode of death, and thus should be considered for ICD only if they are candidates for cardiac resynchronisation therapy
Ischaemic cardiomyopathy (LVEF ≤40%) with non-sustained VT and inducible sustained VT during electrophysiological testing
Ischaemic cardiomyopathy (LVEF ≤30% and New York Heart Association class I symptoms)
Hypertrophic cardiomyopathy (HCM) with high-risk features such as family history of sudden death from HCM; massive left ventricular hypertrophy (wall thickness ≥30 mm); unexplained syncope; left ventricular systolic dysfunction; left ventricular apical aneurysm; extensive late gadolinium enhancement on cardiovascular MRI; or frequent, longer, and faster runs of non-sustained VT.[8][9] Other clinical features that are utilised in calculating sudden cardiac death risk in HCM include age, left atrial diameter, left ventricular outflow tract obstruction, and exercise blood pressure response
Congenital arrhythmia syndromes, including symptomatic patients with long QT syndrome, Brugada syndrome, and catecholaminergic polymorphic VT with high-risk features.
Physical examination
The physical examination in a patient with suspected VT should include an assessment of the patient's level of consciousness, airway stability, breathing, and circulatory support. This will help determine, once VT is confirmed, whether to cardiovert the patient immediately or to attempt pharmacological therapy first.
Signs of airway compromise may include stridor and/or apparent obstruction to breaths delivered during rescue breathing.
Palpation of the carotid or femoral pulse may be necessary to determine the heart rate if the patient is relatively hypotensive (e.g., systolic BP <90 mmHg), and can also provide a crude estimate of the cardiac output (by noting the force of the pulse) if a blood pressure measurement cannot be immediately obtained. Patients with a weak pulse and hypotension are classified as haemodynamically unstable, as are patients with signs of diminished cerebral perfusion (e.g., lightheadedness, dizziness, diminished responsiveness, or unconsciousness) or diminished coronary perfusion (e.g., chest discomfort, dyspnoea). Again, these findings do not always help distinguish VT from SVT.
ECG
The ECG is crucial to the diagnosis of a VT. VT is defined by the presence of a wide complex tachycardia (QRS 120 milliseconds or greater) at a rate of 100 bpm or greater. However, it is important to recognise that not all wide complex tachycardias are due to VT. When evaluating the patient with a wide complex tachycardia it is essential to distinguish VT from SVT that conducts with aberrancy or with pre-excitation, as these conditions are managed very differently.
When available, review of the patient's baseline ECG provides important clues to determining the origin of a wide complex arrhythmia.
Evidence supporting a diagnosis of an SVT includes:
Pre-existing pre-excitation of a similar morphology to the wide complex arrhythmia; and/or
Baseline bundle branch block that resembles the wide complex tachycardia.[22]
A stepwise approach has been proposed that uses 4 steps to differentiate VT from SVT with aberrancy.[23] These steps are:
1. Absence of an RS complex in any precordial lead confirms diagnosis of VT
2. If RS complex is present in step 1, measure the QRS onset to nadir of S wave:
a. R-to-S interval of >100 milliseconds confirms diagnosis of VT
3. If R-to-S interval is <100 milliseconds: examine ECG for atrioventricular (AV) dissociation. If present, diagnosis is VT.
4. If no AV dissociation, examine QRS complex in V1 and V6:
a. with right bundle branch block QRS morphology:
i. in lead V1: monophasic R, QR, or RS favours VT
ii. in lead V6: R/S ratio of <1 favours VT; QS, QR, or monophasic R favours VT
b. with left bundle branch block QRS morphology:
i. in either lead V1 or V2: R >30 milliseconds, R-to-S (nadir) interval of >60 milliseconds or a notched S wave favours VT
ii. in lead V6: QR or QS favours VT
c. Note: both V1/V2 and V6 criteria need to favour VT for the diagnosis to be made using this step.
If none of the steps above favours VT, a diagnosis of SVT is made.
AV dissociation can manifest as dissociated P waves (usually best seen in lead V1) or the presence of capture beats and fusion beats. Fusion beats are changes to the QRS complex that arise when the native rhythm competes or fuses with a cycle of VT. The fusion beat demonstrates a distinct morphology from that of the other QRS complexes in an otherwise monomorphic VT and is intermediate between the wide complexes and the patient's baseline QRS morphology. A capture beat is an ECG phenomenon characterised by a narrow QRS complex that occurs during a run of VT; the capture beat results from a sinus beat temporarily penetrating the VT circuit. The capture beat occurs at an earlier RR interval than would otherwise be expected by the VT cycle length. When evaluating a patient with an undiagnosed wide complex tachycardia, the presence of capture or fusion beats proves that the arrhythmia is VT and not SVT with aberrancy.
Evidence that suggests the arrhythmia is more likely to be from the ventricle includes ventricular premature contractions of similar morphology to the tachycardia present on the baseline ECG.[22]
ECG evidence of previous myocardial infarction also favours a diagnosis of VT.
Another algorithm using only lead aVR for differentiating VT from SVT has been reported, with superior accuracy.[24] Lead aVR was analysed for:
1. Presence of an initial R wave
2. Width of an initial r or q wave >40 milliseconds
3. Notching on the initial downstroke of a predominantly negative QRS complex
4. Ventricular activation-velocity ratio (vi/vt), where v is the vertical excursion recorded during the initial (vi) and terminal (vt) 40 milliseconds of the QRS complex.
The finding of any of the above results in a diagnosis of VT.
Other ECG evidence supporting a diagnosis of VT includes:
QRS duration: >140 milliseconds with right bundle branch block morphology, or QRS duration >160 milliseconds with left bundle branch block morphology (these criteria do not apply to patients treated with anti-arrhythmic drugs)
The presence of a right superior axis or left bundle branch block morphology and any right axis. The absence of extreme axis deviation does not imply a supraventricular origin of the tachycardia.[25]
In 2022, another simple algorithm was published, called the Basel algorithm. The Basel algorithm demonstrated high sensitivity, specificity, and accuracy in diagnosing VT using the 12-lead ECG and history. VT was diagnosed in the presence of at least two of the following criteria: 1) clinical high-risk features (i.e., history of myocardial infarction, or history of congestive heart failure with ejection fraction 35% or less, or in the presence of an ICD or biventricular ICD); 2) lead II time to first peak >40 milliseconds; and 3) lead aVR time to first peak >40 milliseconds. If zero or one criterion was met, then the diagnosis was SVT.[26]
The baseline ECG should also be examined carefully for evidence of:
QT interval prolongation [Figure caption and citation for the preceding image starts]: Prolonged QT: QTc 510 ms (HR 69 and QT 476)From the collection of Prof Sei Iwai; used with permission [Citation ends].
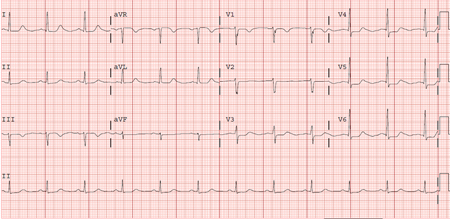
Brugada syndrome: a disorder characterised by cardiac conduction abnormalities, which leads to a characteristic J-point elevation and downwards-sloping ST segment elevation in the right pre-cordial lead that can lead to sudden death due to polymorphic VT. A mutation in the SCN5A (sodium channel) gene has been implicated, but it is present in only a minority of patients. SCN10A has also been identified as a major susceptibility gene for Brugada syndrome.[12]
Arrhythmogenic right ventricular cardiomyopathy (ARVC): a genetic disorder characterised by VT, sudden death, and progressive heart failure. In this disease, various regions of the right (and occasionally the left) ventricular muscle are replaced by deposition of fat and fibrotic tissue. Patients with ARVC frequently manifest right ventricular conduction delay on ECG; the finding of an epsilon wave in lead V1 is a specific, although not sensitive, sign for the disease. An epsilon wave represents late activation of a region of the ventricular myocardium, with delay being due to the presence of fatty infiltration and fibrosis.
Although it is tempting to imagine that the rate of the tachycardia suggests whether or not it is from the ventricle, the tachycardia rate is not helpful in diagnosing the location of the tachycardia. Likewise, the regularity of the tachycardia generally is not helpful to classify its origin, although gross irregularity suggests the possibility of atrial fibrillation with aberrancy or pre-excitation.
Electrolytes and cardiac enzymes
Electrolyte abnormalities (particularly hypokalaemia and hypomagnesaemia) may incite and/or contribute to VT. When time and the patient's condition permit, blood electrolytes should be investigated and any abnormalities of these electrolytes should be corrected.
In situations where ischaemia or infarction is the suspected mechanism of the VT, myocardial biomarker assays (especially troponin) provide useful confirmatory information.
Imaging studies
Given the widely varying prognosis and management strategies for idiopathic VT and VT associated with structural heart disease, it is crucial to establish the presence or absence of structural heart disease in a patient presenting with VT. Echocardiogram is an efficient initial way to determine the presence or absence of structural heart disease in addition to quantifying systolic function.
In patients suspected of having arrhythmogenic right ventricular cardiomyopathy, cardiac MRI is indicated to evaluate for the presence of fibro-fatty infiltration of the right ventricle and for right ventricular dysfunction.
Stress testing and cardiac catheterisation
Stress testing and/or cardiac catheterisation should be considered to establish the presence of coronary artery disease (CAD), to exclude the possibility of asymptomatic/silent ischaemia, especially among patients with risk factors for CAD.
In patients suspected of having the inherited condition catecholaminergic polymorphic VT, diagnosis can be made based on the presence of bidirectional or polymorphic ventricular arrhythmias under conditions of increased sympathetic activity (e.g., following stress test) in young patients who otherwise have structurally normal hearts and normal baseline ECGs.[27] Diagnosis can also be made based on genetic testing for mutations in the genes encoding the cardiac ryanodine-calcium release channel (RyR2) and, in some patients, mutations in the genes encoding cardiac calsequestrin (CASQ2).[16][17]
Electrophysiological (EP) testing
The determination of which patients would benefit from an invasive EP study is made in consultation with an electrophysiologist.[7] It may be required to distinguish VT from SVT with aberrancy in cases where the surface ECG cannot establish a diagnosis. The EP study may also be used to establish the mechanism of VT in patients with structurally normal hearts, and in patients with left ventricular ejection fraction >35% or with unexplained syncope.[28] An EP study may be helpful in such cases in determining whether or not a given patient would benefit from a prophylactic ICD.[7]
Genetic testing
Genetic testing is available for inherited cardiovascular diseases including long QT syndrome, short QT syndrome, Brugada syndrome, hypertrophic cardiomyopathy, and catecholaminergic polymorphic VT.[16][17] Consultation with a medical geneticist is useful when deciding which patients should undergo genetic testing.[16][17] Provision of genetic counselling is an essential part of care for any patients who agree to genetic testing.[16][17]
Use of this content is subject to our disclaimer