Aetiology
The aetiology of DKD is multifactorial, with the most important factors being extent and duration of hyperglycaemia, and hypertension.[4][10][11] Other factors that are thought to increase likelihood of DKD, or to increase its progression, are glomerular hyperfiltration, smoking, obesity, physical inactivity, dyslipidaemia, proteinuria, and high dietary content of protein and fat.[4][10][12][13] Genetic susceptibility appears to be a prerequisite to the development of DKD.[14][15]
Pathophysiology
DKD is caused by both metabolic alterations (hyperglycaemia and possibly hyperlipidaemia) and haemodynamic alterations (systemic and glomerular hypertension), which promote inflammation, endothelial dysfunction, oxidative stress and fibrosis, and glomerular hyperfiltration.[4] Oxidative stress consumes nitric oxide, which prevents flow-mediated dilation (FMD) of blood vessels (endothelial dysfunction), subjecting the endothelium to injury. This leads to production of cytokines, acceleration of inflammation, worsening of blood vessel rigidity due to atherosclerosis, and further impairment of FMD and susceptibility to oxidative stress. From a unified perspective, inflammation, endothelial dysfunction, and oxidative stress are intertwined in a vicious cycle that leads to significant kidney damage and cardiovascular events. One study demonstrated that endothelial dysfunction and inflammation were predictors of progression of DKD in patients with type 2 diabetes and moderately increased albuminuria (previously known as microalbuminuria).[16]
Diabetes mellitus is characterised by high glucose levels and increased glomerular pressure, both of which can cause glomerular mesangium expansion via increased mesangial stretch. Platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-beta) mediate mesangial expansion and fibrosis via the stimulation of matrix protein (collagen and fibronectin) synthesis and decreased matrix degradation. Glucose forms advanced glycation end products (AGEs) by binding irreversibly to proteins. Over years, AGEs form crosslinks, stimulate the release of growth factors such as TGF-beta, and cause fibrosis. Angiotensin II (ATII), elevated in DKD, constricts the efferent arteriole in the glomerulus, causing high glomerular capillary pressures, and also stimulates fibrosis and glomerular inflammation. Mesangial expansion is characteristic of early diabetic glomerulosclerosis and is followed by fibrosis in the late stages. Kimmelstiel-Wilson nodules, areas of mesangial expansion on biopsy, are the hallmark of diabetic glomerulosclerosis and are seen in half of the cases of DKD. Increased glomerular basement membrane width, diffuse mesangial sclerosis, hyalinosis, microaneurysm, and hyaline arteriosclerosis are present in addition to tubular and interstitial changes.[17] Hypertension, via mesangial stretch, can aggravate progression of DKD.[Figure caption and citation for the preceding image starts]: Diabetic kidney disease: mesangial expansion due to increased mesangial matrix and decreased degradation of glycosylated collagenFrom the collection of Dr Raoul Fresco; used with permission [Citation ends].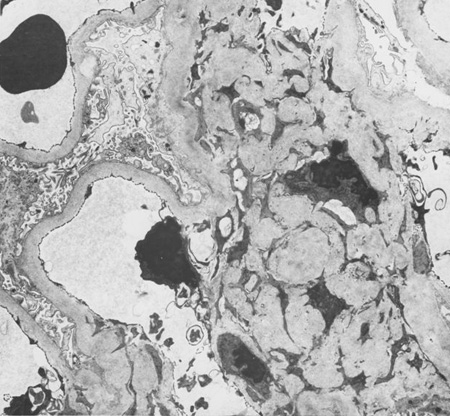 [Figure caption and citation for the preceding image starts]: Diabetic kidney disease: mesangial expansion is usually recognised when it has exceeded 1.5 times the normal mesangial matrixFrom the collection of Dr Raoul Fresco; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Diabetic kidney disease: mesangial expansion is usually recognised when it has exceeded 1.5 times the normal mesangial matrixFrom the collection of Dr Raoul Fresco; used with permission [Citation ends].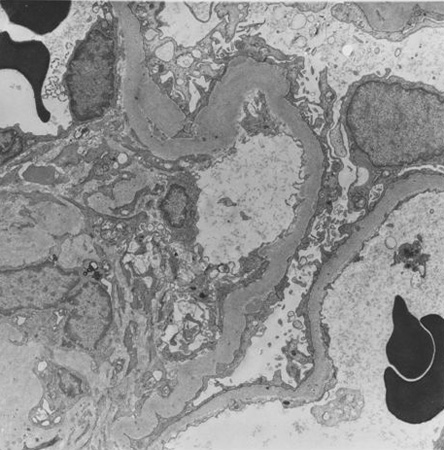 [Figure caption and citation for the preceding image starts]: Diabetic kidney disease: at 5 o'clock - early Kimmelstiel-Wilson nodule, a rounded increase in mesangial matrix that probably originated in relation to a microaneurysmFrom the collection of Dr Raoul Fresco; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Diabetic kidney disease: at 5 o'clock - early Kimmelstiel-Wilson nodule, a rounded increase in mesangial matrix that probably originated in relation to a microaneurysmFrom the collection of Dr Raoul Fresco; used with permission [Citation ends].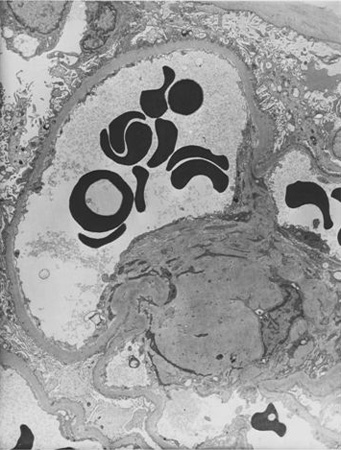 [Figure caption and citation for the preceding image starts]: Diabetic kidney disease: at 12 o'clock - early Kimmelstiel-Wilson nodule, a rounded form of mesangial expansionFrom the collection of Dr Raoul Fresco; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Diabetic kidney disease: at 12 o'clock - early Kimmelstiel-Wilson nodule, a rounded form of mesangial expansionFrom the collection of Dr Raoul Fresco; used with permission [Citation ends].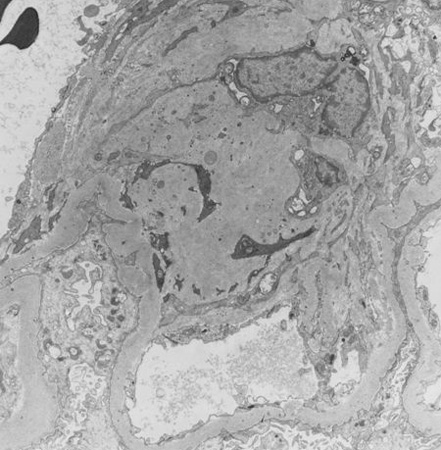
Glomerular filtration rate (GFR) may be increased at the onset of diabetes mellitus (both in those inclined to develop nephropathy and in those who will not develop nephropathy), but once moderately increased albuminuria is present the GFR is usually normal. According to the natural history of the disease, severely increased albuminuria (defined as >30 mg/mmol [>300 mg/g] albuminuria, previously termed macroalbuminuria) occurs before a decline in GFR.[18] However, interventions, especially blood pressure control with drugs that block the renin-angiotensin system and sodium-glucose co-transporter 2 (SGLT2) inhibitors, can alter the natural history. Some patients have a decline in GFR in the absence of severely increased albuminuria.
Due to the overlap in pathophysiology between diabetes, cardiovascular disease (CVD), obesity, and chronic kidney disease (CKD), some groups argue that these conditions should be considered to be on a single spectrum known as cardiovascular-kidney-metabolic (CKM) syndrome. The American Heart Association, notably, has endorsed this terminology and proposed a four-stage model based on a patient's number of risk factors and the presence of overt CVD.[19] It has also developed a series of risk prediction equations, known as PREVENT, for estimating 10- and 30-year CVD risks based on the CKM concept.[20] It is hoped that this new approach will improve screening, prevention, and treatment of CKM risk factors, particularly in people with adverse social determinants of health.[19]
Use of this content is subject to our disclaimer