Approach
Once identified, further imaging and laboratory evaluation are necessary to identify the aetiology and underlying adrenal pathology of the tumour. It is important to determine whether the tumour is hormonally active or inactive, and malignant or benign.[25]
Biochemical studies include standard hormonal evaluation: late night salivary cortisol, or overnight 1 mg dexamethasone suppression test for Cushing syndrome, fractionated plasma metanephrines for phaeochromocytoma, and ratio of plasma aldosterone concentration/plasma renin activity if hypertension or hypokalaemia is present for primary hyperaldosteronism.[26]
Clinical assessment
Patients with an incidental adrenal mass may present with only subtle clinical features of adrenal hormone excess, or their adrenal mass may be hormonally inactive. Therefore, the initial clinical evaluation is focused on assessing the clinical signs and symptoms of hormone overproduction syndromes.[5]
Cushing syndrome: weight gain, central obesity, facial rounding, plethora, supraclavicular fat pads, dorsocervical fat pad, easy bruising, purple striae, proximal weakness, mood changes, infection, irregular menses, acne, hirsutism, hypertension (if virilising or feminising tumour: hirsutism, amenorrhoea or change in libido, gynaecomastia). Individuals with mild autonomous cortisol secretion (MACS), or subclinical Cushing syndrome, may be asymptomatic, or patients may have metabolic alterations such as impaired glucose tolerance, diabetes mellitus, hypertension, or metabolic bone disease without other clinical stigmata.[5][11][27]
Phaeochromocytoma: severe or episodic hypertension, palpitations, pallor, retinopathy, tremor, fever.
Primary hyperaldosteronism: hypertension, muscle cramps/weakness, cardiac arrhythmias, hypokalaemia-induced nephrogenic diabetes insipidus (polyuria, dehydration).
Patients with adrenal cortical carcinoma may present with clinical evidence of:
Mass effect (e.g., abdominal pain)
Adrenal hypersecretion of cortisol (Cushing syndrome), androgens (hirsutism, acne, amenorrhoea, or oligomenorrhoea), oestrogens (gynaecomastia), or, very rarely, aldosterone (hypokalaemia-related symptoms).
Laboratory evaluation
All patients undergo the following work-up.[5][9][21]
Electrolytes: hypokalaemia may suggest hyperaldosteronism; hypokalaemia may be present in Cushing syndrome.
Fasting lipid profile: hyperlipidaemia may be present in Cushing syndrome.
Fasting blood glucose: hyperglycaemia may be present in Cushing syndrome or phaeochromocytoma.
Full blood count: leukocytosis with neutrophilia may occur in Cushing syndrome.
Screening test for Cushing syndrome with late night salivary cortisol or overnight (1 mg) dexamethasone suppression test.[26][27]
Screening for phaeochromocytoma with fractionated plasma metanephrines.
Patients with hypertension should also have plasma renin/aldosterone ratio measured to assess for hyperaldosteronism.
If screening for hormonal activity is positive, further testing is indicated to confirm hormonal autonomy:
For Cushing syndrome, 24-hour urine free cortisol and early morning (9 a.m.) serum corticotropin (ACTH).[26][27]
For phaeochromocytoma, 24-hour urine total metanephrines and fractionated catecholamines.
For hyperaldosteronism, plasma aldosterone concentration on an unrestricted salt diet, 24-hour urinary aldosterone excretion, and saline infusion test (saline suppression test).
Blood levels of androgens (testosterone and dehydroepiandrosterone sulphate) or oestrogens (estradiol) are not routine, but measured when there are signs or symptoms of virilisation in women or feminisation in men.
Venous adrenal sampling confirms that the adrenal mass (and not bilateral adrenal hyperplasia) is the source of aldosterone excess in patients with primary aldosteronism.[5]
European guidelines suggest screening all patients with MACS for hypertension, type 2 diabetes mellitus, and asymptomatic vertebral fractures in the context of untreated osteoporosis.[9]
Imaging
[Figure caption and citation for the preceding image starts]: Workup of an adrenal mass incidentally found on imaging [Abbreviations: CT: computed tomography; CECT: contrast-enhanced CT; MRI: magnetic resonance imaging; CS-MRI: compressed sensing MRI; F-DOPA: 3,4-dihydroxy-6-(18)F-fluoro-l-phenylalanine; FDG: 18-fluoro-2-deoxyglucose; Ga/Cu DOTATATE: ⁶⁸Ga- or ⁶⁴Cu-DOTA-(Tyr3)-octreotate; FNAB: fine-needle aspiration (FNA) biopsy; HU: Hounsfield unit; MIBG: meta-iodobenzylguanidine; NP-59: iodine-131-6-beta-iodomethylnorcholesterol (or radio-iodine-labeled norcholesterol); PET: positron emission tomography; SUVmax: maximum standardised uptake value; US: ultrasound]Adapted by Dr Ka Kit Wong from Mayo-Smith WW et al. J Am Coll Radiol. 2017 Aug;14(8):1038-44; used with permission [Citation ends].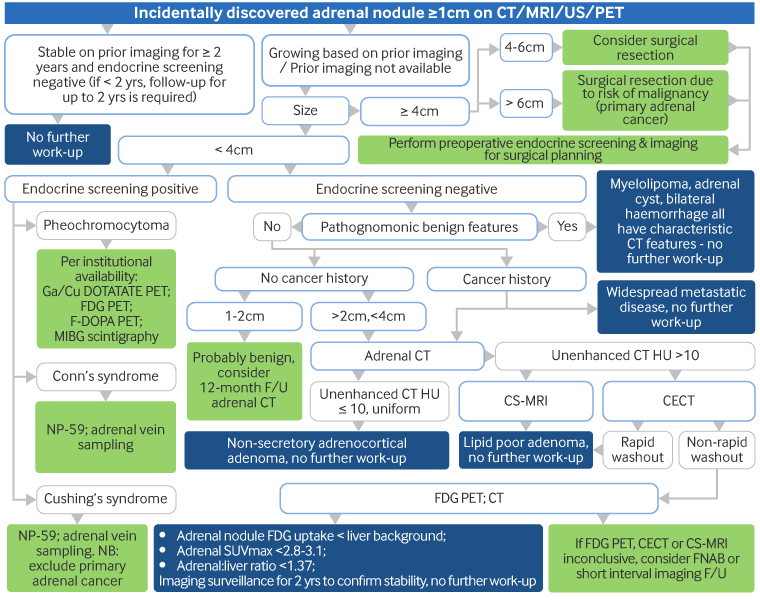
Adrenal incidentalomas may be detected on abdominal computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), or ultrasound for investigation of non-adrenal disorders.
If prior imaging is available then confirming stability in size over a 2-year period is highly predictive of benign aetiology. The size of an adrenal mass should be considered because a larger mass is more likely to be malignant. However, this should not be used in isolation because malignant tumours <3 cm in diameter are not uncommon. The smaller an adrenal carcinoma is at the time of diagnosis, the lower the tumour stage and the better the patient's overall prognosis. Additionally, most adrenal masses >4 cm are benign. The criterion of diameter >4 cm has 90% sensitivity, but only 24% specificity for adrenal cortical carcinoma detection.[5] Size thresholds have been proposed for surgical intervention; size >6 cm is associated with increased risk of adrenal cortical carcinoma requiring surgical resection.[28] Guidelines have recommended surgical resection of adrenal nodules with indeterminate CT features and size >4 or 5 cm due to increased risk for malignancy, although controversy does exist.[21][25]
Imaging modalities may further differentiate adrenal masses.
CT or MRI: to assess size and morphological characteristics of adrenal lesions.[Figure caption and citation for the preceding image starts]: Incidentally discovered, 1.8 cm diameter, right adrenal mass in a 37-year-old man. Abdominal CT (A) depicts the mass as lipid-poor with atypical contrast enhancement; NP-59 scintigraphy done without dexamethasone suppression; (B) demonstrates a concordant pattern of imaging with right > left adrenal accumulation of iodocholesterol on the fifth and sixth days post-injection, compatible with a benign aetiologyDeGroot LJ, Jameson JL, eds. Endocrinology, 5th ed. Philadelphia, PA: Elsevier; 2006; used with permission [Citation ends].
Decisions regarding surgery should take into account not only size but also the imaging phenotype of the mass, as well as the patient's age and any co-existing conditions.[9][21][28]
On unenhanced CT, benign adrenal adenomas (lipid-rich adenomas) typically have ≤10 Hounsfield units attenuation characteristics.[29] If the adrenal lesion has >10 Hounsfield units attenuation, the diagnosis is uncertain (it may represent a lipid-poor adenoma, an adrenocortical carcinoma, adrenal metastasis, or phaeochromocytoma). In this case, a contrast-enhanced CT scan is performed to assess the washout of initial enhancement in the lesion; adrenal adenomas display ≥60% enhancement washout at 15 minutes (sensitivity 88%, specificity 96% for diagnosis of benign adrenal lesion).
Chemical shift MRI is indicated if the adrenal incidentaloma is discovered during MRI study, or if the adrenal mass is indeterminate on contrast-enhanced CT and further characterisation is required. MRI can confirm fat content in lipid-rich adenomas with high specificity.[30]
Functional imaging studies
Mainly performed in patients with biochemical evidence of adrenal, cortical, and medullary hypersecretion to assist surgical planning.
In patients with biochemical evidence of hypersecretory endocrine syndromes and indeterminate CT or MRI findings, functional radionuclide imaging enables further characterisation of the adrenal mass.
Cortical imaging: Radioiodine-labeled norcholesterol (NP-59) scintigraphy
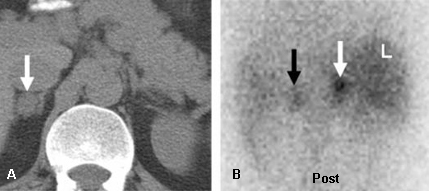
In patients with laboratory evidence of hypercortisolism or hyperaldosteronism, and no history of cancer, functional radionuclide imaging should start with NP-59 scintigraphy. In this group, unilateral uptake on NP-59 scintigraphy within an adrenal mass virtually excludes adrenal malignancy due to primary adrenocortical cancer or metastatic disease. It can also determine laterality of excessive function.[31][Figure caption and citation for the preceding image starts]: Left adrenal aldosteronoma depicted with dexamethasone suppression NP-59 imaging: 57-year-old woman with biochemical evidence of hyperaldosteronism and a left adrenal mass. Abdominal CT (A) demonstrates a 2 cm left adrenal mass (black arrow), anterior and posterior abdominal NP-59 scans (B and C) on the third day post-injection; anterior and posterior abdominal NP-59 scans (D and E) on the fifth day post-injection; abnormal left adrenal uptake (black arrows) occurs early, before day 5 post-injection (B and C); normal uptake in liver (L), bowel (B), and gallbladder (GB)Rubello D, Bui C, Casara D, et al., Eur J Endocrinol. 2002 Jul;147(1):13-28; used with permission [Citation ends].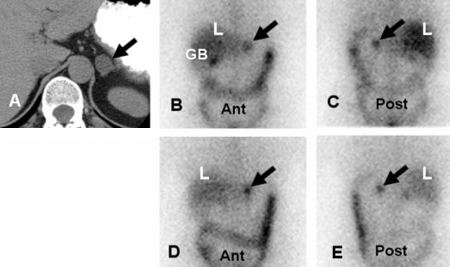
Targeted molecular imaging involving inhibitors of 11β-hydroxylase, including ¹¹C-etomidate and ¹¹C-, ¹⁸F-, and ¹²⁴I-metomidate, is an emerging area. Additionally, chemokine receptor 4-directed imaging with ⁶⁸Ga-Pentixafor has been explored, and early studies involving individuals with haematological malignancies suggest success.[32] Efforts to improve NP-59 with a PET radioisotope 18F-NP-59 (FNP-59) are also a developing area.
Medullary imaging: Metaiodobenzylguanidine (MIBG) scintigraphy
An MIBG scan can be used to assess for phaeochromocytoma.
123-I or 131-I MIBG scintigraphy identifies tumours of chromaffin origin (adrenal medulla and neural crest tumours). This test is used for localisation of sources of hypercatecholaminaemia (intra-adrenal/extra-adrenal/metastatic/familial phaeochromocytomas) and other neuro-endocrine neoplasms (neuroblastomas and paragangliomas). It is indicated when CT or MRI is indeterminate for phaeochromocytoma. One meta-analysis found that MIBG had sensitivity of 94% and specificity of 92% for phaeochromocytoma.[33] In patients that have a positive scan and metastatic disease, MIBG therapy (iobenguane I-131) could be an option. If the MIBG scan is negative, it should be followed by an 18-fluoro-2-deoxyglucose PET scan or a DOTATATE scan. One emerging area in adrenomedullary imaging involves improving 123-I-MIBG scintigraphy using a PET radiotracer. Imaging with 18F-meta-fluorobenzylguanidine has been successful in early trials in patients with neuroendocrine tumours.[34] Another tracer, 3-[18F]fluoro-para-hydroxyphenethylguanidine, has shown promising results for visualisation of functional and non-functional metastatic phaeochromocytoma and paraganglioma.[35]
[Figure caption and citation for the preceding image starts]: Meta-iodobenzylguanidine (MIBG) scan demonstrating intensely focal tracer activity in the left adrenal gland consistent with phaeochromocytoma in a 56-year-old woman with HTN, elevated plasma noradrenaline (norepinephrine) levels, and a 3 cm left adrenal mass seen on cross-sectional anatomical imagingAvram A, Fig LM, Gross MD. Semin Nucl Med. 2006 Jul;36(3):212-27; used with permission [Citation ends].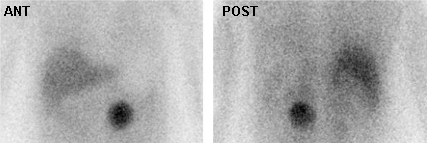 [Figure caption and citation for the preceding image starts]: Malignant, metastatic phaeochromocytoma demonstrated by 123-I-meta-iodobenzylguanidine (MIBG) and CT in a 31-year-old woman after bilateral adrenalectomy and persistent HTN complicated by renal insufficiency and recent development of superior vena cava (SVC) obstruction; (A) anterior chest and abdomen scan. L = normal liver uptake while arrows depict multiple, abnormal foci of 123-1-MIBG in metastatic phaeochromocytoma deposits in the mediastinum and para-aortic regions; (B) chest CT identifies the superior mediastinal mass responsible for SVC obstruction (white arrow)Rubello D, Bui C, Casara D, et al. Eur J Endocrinol. 2002 Jul;147(1):13-28; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Malignant, metastatic phaeochromocytoma demonstrated by 123-I-meta-iodobenzylguanidine (MIBG) and CT in a 31-year-old woman after bilateral adrenalectomy and persistent HTN complicated by renal insufficiency and recent development of superior vena cava (SVC) obstruction; (A) anterior chest and abdomen scan. L = normal liver uptake while arrows depict multiple, abnormal foci of 123-1-MIBG in metastatic phaeochromocytoma deposits in the mediastinum and para-aortic regions; (B) chest CT identifies the superior mediastinal mass responsible for SVC obstruction (white arrow)Rubello D, Bui C, Casara D, et al. Eur J Endocrinol. 2002 Jul;147(1):13-28; used with permission [Citation ends].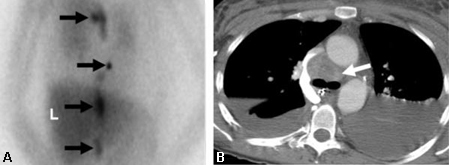
⁶⁸Ga-DOTA-(Tyr3)-octreotate (DOTATATE) PET
Somatostatin expression imaging using DOTATATE can be an effective alternative agent for imaging sympathomedullary neoplasms or neuroendocrine tumours. One small cohort study suggests DOTATATE can offer improved image quality and sensitivity compared to MIBG.[36] It can also be effective in imaging phaeochromocytomas that have minimal or moderate rates of increased glycolysis, which can make FDG imaging challenging. Similar to MIBG, if significant uptake is seen, therapeutic radiotherapy with lutetium Lu 177 dotatate may be employed in patients with metastatic disease.
18-Fluoro-2-deoxyglucose (FDG) PET
In patients with a history of malignancy, 18-FDG PET/CT should be initially performed, and if negative could be followed by NP-59, MIBG, or DOTATATE scans.[25][37]
18-FDG PET is useful for the metabolic characterisation of adrenal lesions (benign versus malignant), which may assist in determining whether surgical resection is warranted due to suspicion for malignancy.[38] 18-FDG PET or PET/CT is used in patients with a history of malignancy, and may be considered in patients in whom CT or MRI is inconclusive.[25]
In patient groups with and without history of cancer, 18-FDG PET has reported sensitivity between 74% to 100%, and specificity 66% to 100%, for characterising benign versus malignant masses.[39] False-negative results occur due to small size, tumours with low FDG-avidity (eg., renal cell cancer), necrosis, and prior chemotherapy. False positives occur due to overlap of metabolic activity with benign adrenal cortical adenoma, benign phaeochromocytoma, and inflammatory diseases of the adrenals.
Phaeochromocytomas are metabolically active tumours and focally concentrate FDG in approximately 75% of cases, FDG uptake being more common in malignant phaeochromocytoma than in benign phaeochromocytoma. Some phaeochromocytomas that poorly concentrate MIBG are generally well visualised with FDG, and most phaeochromocytomas that fail to accumulate FDG are detected with MIBG; therefore, the 18-FDG PET or PET/CT scan is done when the MIBG scan is negative.
Patients with cancer are more likely to already have had 18-FDG PET/CT as part of their staging or re-staging work-up. Otherwise, for patients with cancer, if widespread metastases are already present then further work-up of an adrenal nodule may not be necessary.
[Figure caption and citation for the preceding image starts]: 18-fluoro-2-deoxyglucose (FDG) PET-CT: adrenal adenoma. 46-year-old man with squamous cell carcinoma of the neck (TxN1 tumour) status post-external beam radiotherapy; restaging PET-CT demonstrates low-attenuation, non-FDG avid, 3.7 cm left adrenal nodule consistent with adrenal adenoma; FDG PET study excluded distant metastasis to the adrenal glandGross MD, Avram A, Fig LM, et al. Eur J Nucl Med Mol Imaging. 2007 Apr;34(4):547-57; used with permission [Citation ends].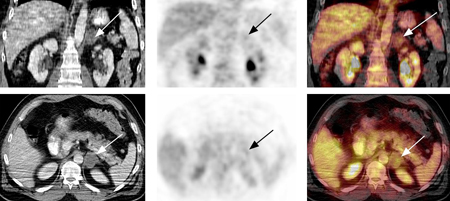
Fluorine-18-L-dihydroxyphenylalanine (18F-DOPA) PET
Performed when both MIBG and 18-FDG PET scans are negative, but there is persistent biochemical evidence for phaeochromocytoma (e.g., elevated plasma fractionated plasma metanephrines).
CT-guided fine needle aspiration (FNA) biopsy
Performed for diagnostic evaluation of adrenal masses in patients with a history of cancer (particularly lung, breast, and kidney) and no other metastases, in order to differentiate between adrenal tissue and non-adrenal tissues (e.g., metastases or infection).
Phaeochromocytoma should always be excluded before the procedure in order to avoid a hypertensive crisis.
CT-guided FNA biopsy is not routinely performed because of the risk of:
procedure-related complications (2.8%; including abdominal pain, adrenal haematoma, haematuria, pancreatitis, pneumothorax, formation of an adrenal abscess)
risk of needle tract metastasis (0.3% of subjects with long-term follow-up), and
high rate of false-negative results.
Generally FNA biopsy is not performed for suspected adrenocortical carcinoma due to concerns regarding seeding of malignancy along the needle tract. If there is a high suspicion, surgical resection is indicated.
FNA biopsy is not usually performed for suspected granulomatous disease, although this is a known cause of false-positive 18-FDG PET results.
FNA biopsy may be required for diagnosis of adrenal nodules/masses still indeterminate for malignancy after imaging evaluation.
If tissue sampling is not indicated, then a strategy of short interval imaging follow-up (at 6 to 12 months) may be pursued.
Other tests
Adrenal vein sampling may also be used for lateralisation of cortisol- or aldosterone-secreting adrenal nodules.
Follow-up
After initial diagnostic evaluation, follow-up of adrenal incidentalomas >1cm includes imaging evaluation at 6 to 12 months to assess for mass enlargement. Lesions that have been stable for 2 years or more can generally be considered to be benign, further radiological follow-up is not required.[25] Biochemical testing annually (overnight 1 mg dexamethasone suppression test and fractionated plasma metanephrines) is performed yearly for 4 years to exclude emergence of subclinical hormonal hypersecretion.[1]
Patients with an adrenal incidentaloma are at risk for tumour growth and development of hormonal alterations. A long-term follow-up study (12-120 months, median 25.5 months) of 64 patients with incidental adrenal masses revealed that cumulative risk of developing endocrine abnormalities was 17% at 1 year, 29% at 2 years, and 47% at 5 years; cumulative risk of mass enlargement was 6% at 1 year, 14% at 2 years, and 29% at 5 years.[40] Rapid growth rate (>2 cm per year) is suggestive of aggressive malignancy (e.g., adrenocortical carcinoma), while stability or slow growth rate (0.5 cm to 1 cm per year) suggests a benign process.[2]
Further follow-up may not be warranted for adrenal tumours that remain stable after 2 imaging studies done at least 6 months apart, and do not exhibit hormonal hypersecretion over 4 years.
Use of this content is subject to our disclaimer