Approach
Whipple's disease has a broad differential as a result of its multisystemic involvement and the absence of specific clinical features. The diagnosis should be considered in all patients with treatment-resistant arthralgia in combination with GI problems and/or neurological signs and enhanced inflammatory parameters. Definitive diagnosis is confirmed by histology and polymerase chain reaction (PCR).
Clinical evaluation
The typical presentation is an acute GI illness with fever, diarrhoea, and weight loss. Features of malabsorption such as steatorrhoea, oedema, fatigue, and lethargy may also be present. There may be progression to a severe wasting syndrome with abdominal lymphadenopathy and abdominal pain. Some patients present with seronegative migratory arthralgia of the large joints or, less often, with a non-deforming oligoarthritis or polyarthritis. Articular problems have been demonstrated to precede the onset of typical GI symptoms by an average of 8 years.[48] Further clinical presentations include anaemia, which is present in most patients, and skin darkening in nearly one half of patients.[11][49]
About 10% to 40% of all patients with Whipple's disease present with neurological manifestations, and, in patients with advanced stages of GI manifestations, CNS presentation can be found in 31%.[14][50][51][52][53] Cognitive dysfunction such as psychiatric signs (e.g., anxiety, depression, hypomania, psychosis, change in personality), memory impairment, dementia, myoclonic signs, decreasing level of consciousness, or confusion is the most common abnormality during CNS disease.
Disturbances of ocular movements are a pathognomonic sign: in particular, progressive supranuclear ophthalmoplegia in conjunction with oculomasticatory myorhythmia (OMM) and oculofacioskeletal myorhythmia (OFSM). Oculomasticatory and oculofacioskeletal myorhythmias are defined as pendular vergence oscillations (PVOs) of the eyes (rhythmic, smooth, convergent eye movements) synchronous with myorhythmias (regular repetitive contractions) of the masticatory, facial, and pharyngeal muscles. OMM is defined as PVOs with masticatory, facial, and pharyngeal myorhythmia. OFSM is defined as PVOs with myorhythmia of non-facial skeletal muscles.[50][52] Further neurological symptoms that may be present include headache, insomnia, epilepsy, focal cerebral lesions, ataxia, seizures, and meningitic features. In some cases, the spinal cord or peripheral nerves may be involved. Other rare neurological signs include nystagmus, upper motor neuron dysfunction (brisk reflexes, extensor plantar responses, weakness predominating in arm extensors and leg flexors, hypertonia), hypothalamic involvement (e.g., amenorrhoea, polydipsia, hyperphagia, decreased libido), hemiparesis, and cranial nerve involvement.[53][54]
Clinical CNS manifestation may be the initial symptom of the disease.[55][56][57] In the absence of neurological symptoms, PCR analysis of CSF reveals CNS infection in about 50% of patients prior to treatment.[21]
Investigations
Using a combination of several classic and alternative investigations in a hierarchical scheme to reach a final diagnosis is recommended. This scheme should be applied to all patients, given that, in the absence of GI symptoms, duodenal biopsies can still reveal a positive histology.[17][Figure caption and citation for the preceding image starts]: Recommended hierarchy for the diagnosis of Whipple's diseaseFrom the collection of Dr Verena Moos [Citation ends].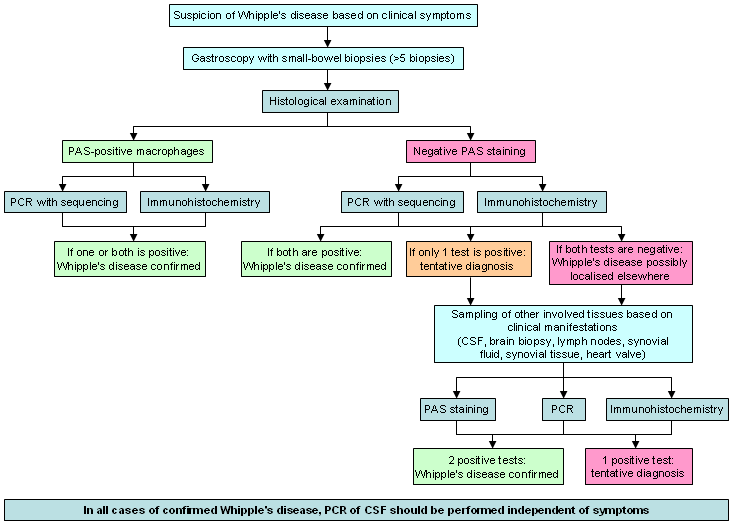
Upper GI endoscopy
Upper GI endoscopy of the small intestine is the preferred first diagnostic test.[50][52] Duodenal mucosa may appear macroscopically pale yellow with clumsy and dilated villi and enlarged lymph vessels. Only histological examination of duodenal biopsies enables the assured diagnosis of Whipple's disease. To avoid sampling errors, at least 5 biopsies should be taken from various sites of the duodenum.
Histology
The histology of the lamina propria in patients with Whipple's disease is characterised by foamy macrophages that contain large amounts of cytoplasmic diastase-resistant periodic acid-Schiff (PAS)-positive particles and are negative for Ziehl-Neelsen staining.[Figure caption and citation for the preceding image starts]: Periodic acid-Schiff-positive macrophages and lymphangia in the duodenal mucosaFrom the collection of Professor Christoph Loddenkemper, Department of Pathology, Charité, CBF, Berlin [Citation ends].
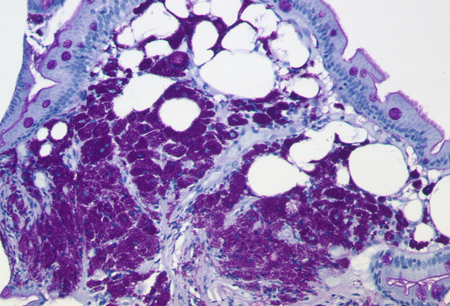 However, about 10% to 15% of patients have only minimal or no GI symptoms.[58][59][60][61] Because PAS-staining of duodenal biopsies may be negative in such cases, biopsies should be obtained from other organs that are involved. For example, PAS-positive cells can often be found in:
However, about 10% to 15% of patients have only minimal or no GI symptoms.[58][59][60][61] Because PAS-staining of duodenal biopsies may be negative in such cases, biopsies should be obtained from other organs that are involved. For example, PAS-positive cells can often be found in:Lymph nodes (especially patients with lymphadenopathies)
CSF
Brain biopsies of patients with CNS disease
Synovial fluid, synovial tissue, or soft-tissue biopsies in patients with arthritis or spondylodiscitis
Cardiac valves of patients with culture-negative endocarditis
Biopsies of skin, liver, muscle, or eye (rare).
Immunohistochemistry
Histological examination based on PAS-staining can be improved by specific immunohistochemistry using polyclonal antibodies against Tropheryma whipplei.[64][65] Because immunohistochemistry is more sensitive, it allows specific and assured identification of T whipplei in tissues that do not stain PAS-positive.[65]
In CNS disease, PAS-staining may be non-specific because of PAS-positive intraneuronal polyglucosan bodies,[66] abnormal glycogen,[67] or human prion protein,[68] which can be misinterpreted as T whipplei inclusions. In these cases immunohistochemistry should be used.
PCR
Healthy carriers may have a positive PCR in specimens from tissues or fluids that are in contact with the environment, but do not have Whipple's disease. Consequently, this method is not suitable for screening, and the detection of T whipplei using PCR should only be applied to patients with established clinical suspicion for Whipple's disease. Because PCR is a very sensitive method prone to false-positive results as a result of contamination, the use of PCR techniques should be limited to accredited laboratories, and the amplified DNA should be verified by sequencing.[69][70] However, PCR is the method of choice for sterile body fluids such as synovial fluid or peritoneal effusions.
Because asymptomatic CNS colonisation is common, CSF sampling and PCR testing for T whipplei should be performed in all patients with a confirmed diagnosis before antibiotic treatment is started.
In patients with only a tentative diagnosis of Whipple's disease, the recommended antibiotic treatment should not be initiated until the diagnosis of Whipple's disease is confirmed or all possible differential diagnoses are definitely excluded.
Blood tests
During acute Whipple's disease, C-reactive protein and erythrocyte sedimentation rate are usually elevated. Haemoglobin is low, and 91% of patients with classic Whipple's disease have hypoalbuminaemia.[11][14]
Diagnosis is based on clinical suspicion and results of PAS-staining, PCR, and immunohistochemistry on biopsies. If duodenal biopsies stain PAS-positive, and one or both of PCR and immunohistochemistry are positive, then the diagnosis of Whipple's disease can be confirmed.
If duodenal biopsies stain PAS-negative but both PCR and immunohistochemistry are positive, then the diagnosis can be confirmed. If, however, only one of PCR or immunohistochemistry is positive, then a tentative diagnosis is made. When both PCR and immunohistochemistry are negative, the possibility that Whipple's disease is localised elsewhere should be considered. Biopsies or fluids should be taken from sites or organs that are also involved. PAS-staining, PCR, and immunohistochemistry should be performed on these new samples. If 2 tests are positive, then the diagnosis is confirmed; if only 1 test is positive, then a tentative diagnosis is made.
Emerging tests
Tests occasionally used to confirm diagnosis include electron microscopy, culture, and serology. These tests are not routinely used. However, if the diagnosis is only tentative, 1 of these additional tests could be applied to ensure the diagnosis. Culture of T whipplei from PCR-positive CSF is a suitable confirmation of diagnosis. Electron microscopy can be used to distinguish T whipplei from Mycobacterium avium-intracellulare, which causes similar intestinal symptoms.
Use of this content is subject to our disclaimer