Investigations
1st investigations to order
ultrasound (US)
Test
Offer a transvaginal ultrasound (TVUS) as the first-line investigation in all patients presenting with symptoms suggestive of adenomyosis (in particular, significant dysmenorrhoea and/or menorrhagia and/or a diffusely enlarged bulky, tender, globular uterus on clinical examination).[2][3][49][50][51]
TVUS is widely available and is generally well tolerated.[58] TVUS is preferred over transabdominal ultrasound due to superior imaging capabilities, including higher resolution and better image quality, achieved through the utilisation of higher transducer frequencies and the elimination of beam deformation caused by the ventral abdomen.[60]
Two-dimensional (2D) TVUS is the most commonly used imaging modality for diagnosing adenomyosis. Three-dimensional (3D) TVUS allows for better visualisation of the junctional zone; one systematic review of the effectiveness of various non-surgical methods for the diagnosis of adenomyosis found that combining 2D TVUS with 3D TVUS improved specificity from 64% with 2D TVUS alone to 81% when combined with 3D TVUS, without a significant difference in sensitivity (85% compared with 84%).[83]
TVUS should be used to assess for direct and indirect features of adenomyosis.[61][62] Note that the presence of indirect features in the absence of direct features is not conclusive for the presence of adenomyosis.[62]
Direct features of adenomyosis are: cysts within the myometrium, which if present may measure between 1 mm and 5 mm and are a specific ultrasound finding for adenomyosis; hyperechogenic islands; echogenic sub-endometrial lines or buds, which indicate the existence of ectopic endometrial glands and stroma beyond the sub-endometrial layer.[62][63][64]
Indirect features are: a globular uterus; asymmetrical myometrial thickening; fan-shaped shadowing - the 'Venetian blind' appearance with fan-shaped shadowing is a posterior acoustic artifact that can be caused by echogenic heterotopic myometrial tissue; translesional vascularity; ill-defined endomyometrial junction or irregular junctional zone - considered suggestive of adenomyosis if junctional zone is poorly defined, irregular, or interrupted.[62][65]
TVUS is also used to assess: the extent of disease, classified as mild (<25% of myometrium, moderate (25% to 50% of myometrium), or severe (>50% of myometrium); location of disease (anterior wall, posterior wall, left lateral, right lateral, fundus); the size of the largest lesion or affected area; differentiation between focal versus diffuse disease; and uterine layer involvement (junctional zone, middle myometrium, or outer myometrium).[2][49][61] Note that in focal disease, nodular aggregates of endometrial glands and stroma are surrounded by normal myometrium, whereas in diffuse disease there are endometrial glands and stroma distributed throughout the myometrium.[1] One group has proposed that an adenomyotic lesion should be classified as focal if >25% of the lesion is surrounded by normal myometrium and as diffuse if <25% is surrounded by normal myometrium.[1] If there is difficulty distinguishing focal from diffuse disease, the adenomyosis should be classified as diffuse.[1]
In adolescents, or if an adult patient declines TVUS, a transabdominal ultrasound or MRI may be offered as an alternative.[3][50]
Result
may manifest as diffuse or focal myometrial findings (adenomyoma) on imaging; may be a globular enlarged uterus, myometrial echotexture abnormalities, sub-endometrial nodules, cysts, echogenic striations, and an ill-defined or irregular endomyometrial junction; 'Venetian blind' appearance may be noted
Investigations to consider
MRI
Test
May be appropriate:
for interventional treatment planning, due to lower false-positive rates and reduced inter-observer variability[2][50]
following TVUS if there is inconclusive sonographic evaluation of adenomyosis or if there is suspicion of significant concomitant pelvic pathology[2][51]
for women with suspected adenomyosis who decline TVUS or for whom it is unsuitable (e.g., adolescents).[3][49]
MRI has a similar sensitivity to TVUS (reported range: 77% to 78%), but its specificity is higher (reported range: 88% to 93%).[66][67][68] The positive likelihood ratio for adenomyosis (the probability that a positive test would be expected in a patient with the condition divided by the probability that a positive test would be expected in a patient without the condition) is higher with MRI compared with TVUS (11.98 vs. 4.93, respectively).[67] MRI is better able than TVUS to differentiate between adenomyosis and leiomyomas (uterine fibroids).[49][68]
MRI can be used to diagnose adenomyosis by evaluating the thickness of the junctional zone, which appears as a distinct T2 hypointense band separating the T2-hyperintense endometrium and the intermediate-intensity myometrium.[69] T2 hypointense junctional zone thickness greater than 12 mm is indicative of adenomyosis, while a thickness less than 8 mm excludes the disease.[70] T2 hyperintense foci can be observed, representing cystic dilation of the endometrial glands, and are similar to anechoic myometrial cysts seen on TVUS.[63][71] T1 signal hyperintensity can be seen if haemorrhage occurs in these foci, which has high specificity (up to 95%) for adenomyosis.[63][71]
Direct features of adenomyosis that may be seen on MRI include myometrial cysts (sensitivity of 60% and specificity of 96%), adenomyoma (a myometrial mass that does not involve the uterine serosa or junctional zone), and/or external adenomyosis (involvement of the serosa but not the junctional zone).[72][73]
Diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) images are beneficial.[58] DWI can be helpful in differentiating between benign and malignant neoplasms when characterising lesions.[74][75] DCE is useful to distinguish endometrial pathology, such as polyps and tumours, from myometrial lesions such as fibroids, as myometrial lesions are typically hypovascular.[76]
Note that MRI should not be performed during menstruation or the early proliferative phase to avoid pseudo-widening of the junctional zone, which can occur due to decreased signal intensity of the myometrium.[72]
There is limited research associating specific features of adenomyosis on MRI with clinical symptoms and symptom severity.[77]
[Figure caption and citation for the preceding image starts]: Sagittal MRI of a woman's pelvis showing a uterus with adenomyosis in the posterior wall. Gross enlargement of the posterior wall is noted, with many foci of hyperintensityCase courtesy of Dr Varun Babu, Radiopaedia.org. From the case rID: 43504; reproduced under the Creative Commons CC BY-SA 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/ ) [Citation ends].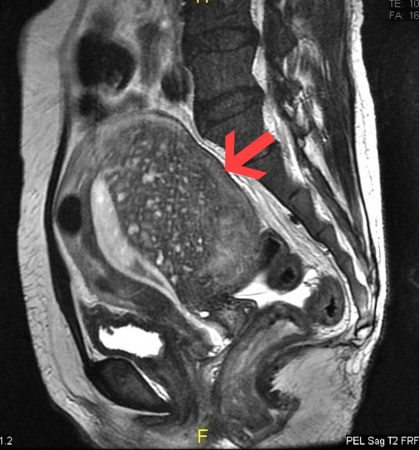
Result
distinct T2 hypointense band separating the T2-hyperintense endometrium and the intermediate-intensity myometrium; T2 hypointense junctional zone thickness >12 mm is indicative of adenomyosis; T2 hyperintense foci may be observed, with or without T1 signal hyperintensity
histological diagnosis following hysterectomy
Test
Histological examination is considered to be the definitive method for confirming a diagnosis of adenomyosis and involves identification of endometrial stroma and glandular tissue within the smooth muscle of the myometrium.[78] It is appropriate for women who have no wish for future fertility and in whom hysterectomy is considered the most appropriate option for managing adenomyosis.[2]
Note that histological diagnosis can be influenced by several factors, including variability in the depth of myometrial invasion, which plays a crucial role in categorising the pathology as 'adenomyosis'.[16] Additionally, the extent of uterine sectioning during tissue sampling and inter-observer variability can also impact the accuracy of the diagnosis.[79]
Result
presence of endometrial stroma and glandular tissue within the smooth muscle of the myometrium confirms the diagnosis
hysterosalpingography (HSG)
Test
Although HSG is not typically used to diagnose adenomyosis, it is often performed on patients with infertility as a part of their work-up and adenomyosis may be incidentally detected.[80]
HSG involves injecting contrast into the endometrial cavity, which fills the cavity and cystic spaces in the glandular endometrial tissue.
Adenomyosis is characterised by heterotopic endometrial foci that extend into the myometrium, which also fills with contrast. As a result, patients with adenomyosis often exhibit a distinct appearance of contrast outpouching into the myometrium at the margins of the endometrial cavity.[80]
Result
contrast outpouchings into the myometrium at the margins of the endometrial cavity
Emerging tests
fertility-sparing biopsy techniques
Test
Fertility-sparing tissue-sampling techniques are under investigation for their potential to obtain diagnostic samples while preserving reproductive potential.[2] Directed tissue-sampling techniques are reserved for specific scenarios in which confirmatory diagnosis may be of benefit.[2] If laparoscopy is performed for other fertility-sparing reasons and there is a preliminary suspicion of adenomyosis, such biopsy techniques may have a role in providing a confirmatory diagnosis and guiding future surgical treatment recommendations for these patients.[81]
These techniques can be performed either through intrauterine tissue sampling using a hysteroscopic approach or via extrauterine sampling, where a myometrial biopsy is obtained through ultrasound or laparoscopy guidance.[81]
The sensitivity of these tissue-sampling techniques varies significantly, ranging from 22.3% to 97.8%, with the highest sensitivity being reported for laparoscopic-guided uterine biopsy with a 14-gauge needle.[82] The variation in sensitivity may be influenced by factors such as the number and location of biopsies, as well as the technique employed for biopsy collection.[81] The specificity of these techniques is generally higher, ranging from 78.5% to 100%.[81]
Result
presence of endometrial stroma and glandular tissue within the smooth muscle of the myometrium confirms the diagnosis
Use of this content is subject to our disclaimer