General principles
Adenomyosis is a non-cancerous (benign) gynaecological condition characterised by the existence of endometrial-like tissue within the uterine myometrium.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
It is most commonly definitively diagnosed via histopathology in women between 35 years of age and menopause. Approximately 70% to 80% of women who undergo hysterectomy for adenomyosis are in the fourth or fifth decade of life.[9]Taran FA, Stewart EA, Brucker S. Adenomyosis: epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe Frauenheilkd. 2013 Sep;73(9):924-31.
https://www.thieme-connect.de/products/ejournals/html/10.1055/s-0033-1350840
http://www.ncbi.nlm.nih.gov/pubmed/24771944?tool=bestpractice.com
However, studies using magnetic resonance imaging (MRI)-based diagnostic criteria highlight that adenomyosis can be a cause of dysmenorrhoea and chronic pelvic pain in women of all ages, including adolescents.[10]Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006 Sep;86(3):711-5.
https://www.fertstert.org/article/S0015-0282(06)00904-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/16782099?tool=bestpractice.com
[11]Royal College of Obstetricians and Gynaecologists. The initial management of chronic pelvic pain (Green-top Guideline no. 41). May 2012 [internet publication].
https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-initial-management-of-chronic-pelvic-pain-green-top-guideline-no-41
After menopause, oestrogen deficiency may decrease both symptoms and prevalence of adenomyosis; in one study, premenopausal and perimenopausal women had an increased prevalence of surgically confirmed adenomyosis at baseline compared with postmenopausal women not using hormone therapy (prevalence odds ratio [POR] 4.72, 95% CI 3.22 to 6.91 and 3.40, 95% CI 2.10 to 5.51, respectively).[12]Templeman C, Marshall SF, Ursin G, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008 Aug;90(2):415-24.
https://www.fertstert.org/article/S0015-0282(07)01371-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17919609?tool=bestpractice.com
Consider the possibility of adenomyosis if a woman presents with dysmenorrhoea, menorrhagia, dyspareunia, and/or chronic pelvic pain.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[11]Royal College of Obstetricians and Gynaecologists. The initial management of chronic pelvic pain (Green-top Guideline no. 41). May 2012 [internet publication].
https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-initial-management-of-chronic-pelvic-pain-green-top-guideline-no-41
[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
Note, however, that around 30% of patients are asymptomatic and may be diagnosed incidentally following hysterectomy or in women with a history of subfertility and/or adverse pregnancy outcomes.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[8]Bourdon M, Santulli P, Marcellin L, et al. Adenomyosis: an update regarding its diagnosis and clinical features. J Gynecol Obstet Hum Reprod. 2021 Dec;50(10):102228.
http://www.ncbi.nlm.nih.gov/pubmed/34520877?tool=bestpractice.com
Be aware that adenomyosis is one of multiple differential diagnoses to consider if a woman presents with abnormal uterine bleeding (AUB), such as endometriosis, leiomyoma (uterine fibroids), polyps, endometrial hyperplasia, and malignancy.[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
The diagnosis of adenomyosis has traditionally relied on histopathological examination (usually following hysterectomy), but recent interest has shifted towards using transvaginal ultrasound (TVUS) or MRI to make a diagnosis.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
However, universally recognised diagnostic criteria are lacking for all modalities.
History
Patients with symptomatic adenomyosis may present with:
Dysmenorrhoea (present in a reported 95% of cases).[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[17]Isaacson K, Loring M. Symptoms of adenomyosis and overlapping diseases. Semin Reprod Med. 2020 May;38(2-03):144-50.
http://www.ncbi.nlm.nih.gov/pubmed/33352607?tool=bestpractice.com
Abnormal uterine bleeding (AUB).
Approximately 65% of patients with adenomyosis experience menorrhagia.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[17]Isaacson K, Loring M. Symptoms of adenomyosis and overlapping diseases. Semin Reprod Med. 2020 May;38(2-03):144-50.
http://www.ncbi.nlm.nih.gov/pubmed/33352607?tool=bestpractice.com
Menorrhagia is defined based on the patient's perception of excessive blood loss that is heavy enough to interfere with quality of life (physically, socially, emotionally, and/or materially).[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
Menstruation lasting >8 days is also defined as AUB by the International Federation of Gynecology and Obstetrics (FIGO).[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
Dyspareunia (reported by 60%).[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[17]Isaacson K, Loring M. Symptoms of adenomyosis and overlapping diseases. Semin Reprod Med. 2020 May;38(2-03):144-50.
http://www.ncbi.nlm.nih.gov/pubmed/33352607?tool=bestpractice.com
Chronic pelvic pain (reported by 50% to 90%).[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[11]Royal College of Obstetricians and Gynaecologists. The initial management of chronic pelvic pain (Green-top Guideline no. 41). May 2012 [internet publication].
https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-initial-management-of-chronic-pelvic-pain-green-top-guideline-no-41
[52]American College of Obstetricians and Gynecologists. ACOG practice bulletin, no. 218: chronic pelvic pain. Obstet Gynecol. 2020 Mar;135(3):e98-109.
https://aogcr.com/wp-content/uploads/2020/08/Chronic-Pelvic-Pain-ACOG-Practice-Bulletin-2012-2020.pdf
http://www.ncbi.nlm.nih.gov/pubmed/32080051?tool=bestpractice.com
Pressure symptoms (genitourinary or gastrointestinal).[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
In one cross-sectional study of 31 women with adenomyosis, 26% reported abdominal pressure symptoms.[53]Nelsen LM, Lenderking WR, Pokrzywinski R, et al. Experience of symptoms and disease impact in patients with adenomyosis. Patient. 2018 Jun;11(3):319-28.
https://link.springer.com/article/10.1007/s40271-017-0284-2
http://www.ncbi.nlm.nih.gov/pubmed/29197944?tool=bestpractice.com
Be alert to the possibility of adenomyosis in patients who present with unexplained subfertility, infertility, and/or adverse pregnancy outcomes and who may be otherwise asymptomatic.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
In one study of 1015 patients undergoing assisted reproduction techniques following a history of infertility, pregnancy loss, or recurrent implantation failure, 24.4% were found to have sonographic signs of adenomyosis.[54]Puente JM, Fabris A, Patel J, et al. Adenomyosis in infertile women: prevalence and the role of 3D ultrasound as a marker of severity of the disease. Reprod Biol Endocrinol. 2016 Sep 20;14(1):60.
https://rbej.biomedcentral.com/articles/10.1186/s12958-016-0185-6
http://www.ncbi.nlm.nih.gov/pubmed/27645154?tool=bestpractice.com
One systematic review and meta-analysis found that adenomyosis was associated with a higher risk of adverse pregnancy outcomes including preterm delivery (odds ratio [OR] 2.65), pre-eclampsia (OR 4.32), pregnancy-induced hypertension (OR 3.11), caesarean section (OR 2.48), fetal malpresentation (OR 3.05), small for gestational age (OR 2.86), intrauterine growth restriction (OR 3.4), postpartum haemorrhage (OR 2.90), and placental malposition (OR 4.94).[55]Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod Biomed Online. 2021 Jan;42(1):185-206.
https://www.rbmojournal.com/article/S1472-6483(20)30528-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33191131?tool=bestpractice.com
One literature review and meta-analysis assessing IVF/intracytoplasmic sperm injection (ICSI) outcomes found that women with adenomyosis had a reduced likelihood of clinical pregnancy compared with women without adenomyosis (clinical pregnancy rate of 41% in women with adenomyosis vs. 50% in those without).[56]Vercellini P, Consonni D, Dridi D, et al. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014 May;29(5):964-77.
http://www.ncbi.nlm.nih.gov/pubmed/24622619?tool=bestpractice.com
The same study found that miscarriage occurred in 32% of women with adenomyosis compared with 14% of those without adenomyosis.[56]Vercellini P, Consonni D, Dridi D, et al. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014 May;29(5):964-77.
http://www.ncbi.nlm.nih.gov/pubmed/24622619?tool=bestpractice.com
Obtain a detailed history in any patient with suspected adenomyosis.[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
Ensure that this covers:
Symptoms. Enquire about the presence and duration of each presenting symptom, including age of onset, frequency of symptom occurrence, and impact on quality of life. Ask in particular about pelvic pain and/or pressure symptoms.[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
Dysmenorrhoea, dyspareunia, and chronic pelvic pain are cardinal symptoms of both adenomyosis and endometriosis.[11]Royal College of Obstetricians and Gynaecologists. The initial management of chronic pelvic pain (Green-top Guideline no. 41). May 2012 [internet publication].
https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-initial-management-of-chronic-pelvic-pain-green-top-guideline-no-41
In one case-control study comparing symptoms among 255 women undergoing hysterectomy, pain symptoms predicted a higher likelihood of both adenomyosis and fibroids rather than fibroids alone being found on histology.[41]Taran FA, Weaver AL, Coddington CC, et al. Characteristics indicating adenomyosis coexisting with leiomyomas: a case-control study. Hum Reprod. 2010 May;25(5):1177-82.
https://pmc.ncbi.nlm.nih.gov/articles/PMC2854044
http://www.ncbi.nlm.nih.gov/pubmed/20176591?tool=bestpractice.com
Menstrual history. Obtain a full menstrual history, including age of menarche, menstrual bleeding pattern and severity (e.g., clots or flooding), and pain associated with bleeding.[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
Early menarche may be a risk factor for the development of adenomyosis. In one large cohort study of 80,000 women, menarche at or before age 10 was associated with a 59% increase in prevalence of surgically confirmed adenomyosis compared with later age of menarche (prevalence odds ratio [POR] 1.59, 95% CI 1.26 to 2.01).[12]Templeman C, Marshall SF, Ursin G, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008 Aug;90(2):415-24.
https://www.fertstert.org/article/S0015-0282(07)01371-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17919609?tool=bestpractice.com
The relationship between menstrual cycle length and the risk of adenomyosis remains inconclusive, although some studies suggest that shorter menstrual cycles may be associated with a higher risk.[12]Templeman C, Marshall SF, Ursin G, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008 Aug;90(2):415-24.
https://www.fertstert.org/article/S0015-0282(07)01371-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17919609?tool=bestpractice.com
[39]Parazzini F, Vercellini P, Panazza S, et al. Risk factors for adenomyosis. Hum Reprod. 1997 Jun;12(6):1275-9.
http://www.ncbi.nlm.nih.gov/pubmed/9222017?tool=bestpractice.com
[43]Parazzini F, Mais V, Cipriani S, et al. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol. 2009 Apr;143(2):103-6.
http://www.ncbi.nlm.nih.gov/pubmed/19232812?tool=bestpractice.com
Gynaecological history, including any past history of endometriosis or uterine leiomyomas (fibroids).[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
Adenomyosis shares key pathological traits with endometriosis and has been shown to be present in approximately 65% of women with histologically proven endometriosis.[17]Isaacson K, Loring M. Symptoms of adenomyosis and overlapping diseases. Semin Reprod Med. 2020 May;38(2-03):144-50.
http://www.ncbi.nlm.nih.gov/pubmed/33352607?tool=bestpractice.com
[38]Bulun SE, Yildiz S, Adli M, et al. Endometriosis and adenomyosis: shared pathophysiology. Fertil Steril. 2023 May;119(5):746-50.
https://www.fertstert.org/article/S0015-0282(23)00211-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36925057?tool=bestpractice.com
Adenomyosis often occurs alongside leiomyomas; in women with leiomyomas who undergo hysterectomy, the prevalence of adenomyosis in the specimens ranges between 15% and 57%.[39]Parazzini F, Vercellini P, Panazza S, et al. Risk factors for adenomyosis. Hum Reprod. 1997 Jun;12(6):1275-9.
http://www.ncbi.nlm.nih.gov/pubmed/9222017?tool=bestpractice.com
[40]Vercellini P, Parazzini F, Oldani S, et al. Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod. 1995 May;10(5):1160-2.
http://www.ncbi.nlm.nih.gov/pubmed/7657758?tool=bestpractice.com
[41]Taran FA, Weaver AL, Coddington CC, et al. Characteristics indicating adenomyosis coexisting with leiomyomas: a case-control study. Hum Reprod. 2010 May;25(5):1177-82.
https://pmc.ncbi.nlm.nih.gov/articles/PMC2854044
http://www.ncbi.nlm.nih.gov/pubmed/20176591?tool=bestpractice.com
A strong correlation has also been found between adenomyosis and endometrial hyperplasia.[4]Bergholt T, Eriksen L, Berendt N, et al. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod. 2001 Nov;16(11):2418-21.
http://www.ncbi.nlm.nih.gov/pubmed/11679531?tool=bestpractice.com
[39]Parazzini F, Vercellini P, Panazza S, et al. Risk factors for adenomyosis. Hum Reprod. 1997 Jun;12(6):1275-9.
http://www.ncbi.nlm.nih.gov/pubmed/9222017?tool=bestpractice.com
Obstetric history. Ask about any previous pregnancies and outcomes, as well as any history of miscarriage, ectopic pregnancy, pregnancy complications, and infertility (including assisted reproductive treatments and outcomes). Enquire about any previous caesarean sections.
While some studies have reported an association between parity and adenomyosis in hysterectomy patients, results have been inconsistent.[37]Upson K, Missmer SA. Epidemiology of adenomyosis. Semin Reprod Med. 2020 May;38(2-03):89-107.
https://pmc.ncbi.nlm.nih.gov/articles/PMC7927213
http://www.ncbi.nlm.nih.gov/pubmed/33105509?tool=bestpractice.com
One study of 985 women undergoing TVUS revealed a positive correlation between the number of pregnancies and the risk of adenomyosis.[6]Naftalin J, Hoo W, Pateman K, et al. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012 Dec;27(12):3432-9.
http://www.ncbi.nlm.nih.gov/pubmed/23001775?tool=bestpractice.com
Some studies have reported an association between spontaneous and induced abortions and an increased risk of adenomyosis, although the evidence is inconsistent.[39]Parazzini F, Vercellini P, Panazza S, et al. Risk factors for adenomyosis. Hum Reprod. 1997 Jun;12(6):1275-9.
http://www.ncbi.nlm.nih.gov/pubmed/9222017?tool=bestpractice.com
[40]Vercellini P, Parazzini F, Oldani S, et al. Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod. 1995 May;10(5):1160-2.
http://www.ncbi.nlm.nih.gov/pubmed/7657758?tool=bestpractice.com
Spontaneous and induced abortions may cause disruption of the endometrial-myometrial border if the pregnancy lasts longer than 9 weeks, due to peak trophoblast invasion during this time.[39]Parazzini F, Vercellini P, Panazza S, et al. Risk factors for adenomyosis. Hum Reprod. 1997 Jun;12(6):1275-9.
http://www.ncbi.nlm.nih.gov/pubmed/9222017?tool=bestpractice.com
[40]Vercellini P, Parazzini F, Oldani S, et al. Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod. 1995 May;10(5):1160-2.
http://www.ncbi.nlm.nih.gov/pubmed/7657758?tool=bestpractice.com
Note that among parous women, those who have breastfed have been shown in some studies to have a decreased incidence of surgically confirmed adenomyosis compared with those who have never breastfed (POR 0.74, 95% CI 0.62 to 0.88).[12]Templeman C, Marshall SF, Ursin G, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008 Aug;90(2):415-24.
https://www.fertstert.org/article/S0015-0282(07)01371-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17919609?tool=bestpractice.com
History of uterine surgery. Data from two studies conducted among hysterectomy patients suggested a positive association between past uterine surgery and adenomyosis.[9]Taran FA, Stewart EA, Brucker S. Adenomyosis: epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe Frauenheilkd. 2013 Sep;73(9):924-31.
https://www.thieme-connect.de/products/ejournals/html/10.1055/s-0033-1350840
http://www.ncbi.nlm.nih.gov/pubmed/24771944?tool=bestpractice.com
[43]Parazzini F, Mais V, Cipriani S, et al. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol. 2009 Apr;143(2):103-6.
http://www.ncbi.nlm.nih.gov/pubmed/19232812?tool=bestpractice.com
[44]Panganamamula UR, Harmanli OH, Isik-Akbay EF, et al. Is prior uterine surgery a risk factor for adenomyosis? Obstet Gynecol. 2004 Nov;104(5 pt 1):1034-8.
http://www.ncbi.nlm.nih.gov/pubmed/15516398?tool=bestpractice.com
Most studies have found no association between previous caesarean delivery and risk of adenomyosis, although one study did report a modest association.[4]Bergholt T, Eriksen L, Berendt N, et al. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod. 2001 Nov;16(11):2418-21.
http://www.ncbi.nlm.nih.gov/pubmed/11679531?tool=bestpractice.com
[5]Curtis KM, Hillis SD, Marchbanks PA, et al. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am J Obstet Gynecol. 2002 Sep;187(3):543-4.
http://www.ncbi.nlm.nih.gov/pubmed/12237624?tool=bestpractice.com
[43]Parazzini F, Mais V, Cipriani S, et al. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol. 2009 Apr;143(2):103-6.
http://www.ncbi.nlm.nih.gov/pubmed/19232812?tool=bestpractice.com
[45]Trabert B, Weiss NS, Rudra CB, et al. A case-control investigation of adenomyosis: impact of control group selection on risk factor strength. Womens Health Issues. 2011 Mar-Apr;21(2):160-4.
https://pmc.ncbi.nlm.nih.gov/articles/PMC3052973
http://www.ncbi.nlm.nih.gov/pubmed/21269840?tool=bestpractice.com
Family history of AUB, uterine conditions, and bleeding disorders.
Enquire about drugs taken, including herbal remedies and supplements.[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
Use of tamoxifen has been associated with a higher incidence of adenomyosis.[18]Cohen I, Beyth Y, Tepper R, et al. Adenomyosis in postmenopausal breast cancer patients treated with tamoxifen: a new entity? Gynecol Oncol. 1995 Jul;58(1):86-91.
http://www.ncbi.nlm.nih.gov/pubmed/7789896?tool=bestpractice.com
[19]Cohen I, Beyth Y, Shapira J, et al. High frequency of adenomyosis in postmenopausal breast cancer patients treated with tamoxifen. Gynecol Obstet Invest. 1997;44(3):200-5.
http://www.ncbi.nlm.nih.gov/pubmed/9359649?tool=bestpractice.com
Note that use of certain drugs or herbal remedies can cause AUB, including:[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
Anticoagulants (e.g., heparin, warfarin)
Hormonal contraceptives
Non-steroidal anti-inflammatory drugs (NSAIDs)
Ginkgo
Ginseng
Motherwort.
Note that some studies have reported an increased prevalence of adenomyosis in Latina women compared with white women and in black women compared with Hispanic individuals.[12]Templeman C, Marshall SF, Ursin G, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008 Aug;90(2):415-24.
https://www.fertstert.org/article/S0015-0282(07)01371-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17919609?tool=bestpractice.com
[42]Jean-Baptiste H, Tetrokalashvili M, Williams T, et al. Characteristics associated with postoperative diagnosis of adenomyosis or combined adenomyosis with fibroids. Int J Gynaecol Obstet. 2013 Aug;122(2):112-4.
http://www.ncbi.nlm.nih.gov/pubmed/23642890?tool=bestpractice.com
Physical examination
Perform a general physical examination in all patients with suspected adenomyosis, with a focus on excluding other potential causes of AUB. Note:
Body mass index (BMI). Obesity may be a pointer to anovulatory AUB. Evidence is inconsistent on whether a BMI ≥30 kg/m² is associated with a higher risk of adenomyosis.[12]Templeman C, Marshall SF, Ursin G, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008 Aug;90(2):415-24.
https://www.fertstert.org/article/S0015-0282(07)01371-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17919609?tool=bestpractice.com
[43]Parazzini F, Mais V, Cipriani S, et al. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol. 2009 Apr;143(2):103-6.
http://www.ncbi.nlm.nih.gov/pubmed/19232812?tool=bestpractice.com
[45]Trabert B, Weiss NS, Rudra CB, et al. A case-control investigation of adenomyosis: impact of control group selection on risk factor strength. Womens Health Issues. 2011 Mar-Apr;21(2):160-4.
https://pmc.ncbi.nlm.nih.gov/articles/PMC3052973
http://www.ncbi.nlm.nih.gov/pubmed/21269840?tool=bestpractice.com
[48]Taran FA, Wallwiener M, Kabashi D, et al. Clinical characteristics indicating adenomyosis at the time of hysterectomy: a retrospective study in 291 patients. Arch Gynecol Obstet. 2012 Jun;285(6):1571-6.
http://www.ncbi.nlm.nih.gov/pubmed/22193824?tool=bestpractice.com
Signs of polycystic ovary syndrome (PCOS) (e.g., hirsutism and acne).
Signs of thyroid disease (e.g., thyroid nodule).
Signs of insulin resistance (e.g., acanthosis nigricans).
Findings suggestive of a bleeding disorder such as petechiae, ecchymoses, skin pallor, or swollen joints.
Perform a pelvic examination, including:[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
External examination ± speculum examination (perform in all consenting adults and consider in consenting adolescents who are sexually active): inspect for visual abnormalities and to help exclude other pathologies that may be a cause of pelvic pain or bleeding symptoms, such as infection, vaginal lesions, or cervical lesions.
Bimanual examination (perform in all consenting adults and consider in consenting adolescents who are sexually active): a mobile, diffusely enlarged, bulky, and tender uterus is highly suggestive of adenomyosis.[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
Differential diagnosis
The differential diagnosis of adenomyosis is extensive and often presents a clinical challenge. Many manifestations of adenomyosis, including AUB, dysmenorrhoea, and infertility, can result from either adenomyosis alone or other commonly concurrent pathologies.
Keep in mind that adenomyosis commonly co-occurs with other causes of AUB, such as endometriosis, uterine leiomyomas, and endometrial hyperplasia.[4]Bergholt T, Eriksen L, Berendt N, et al. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod. 2001 Nov;16(11):2418-21.
http://www.ncbi.nlm.nih.gov/pubmed/11679531?tool=bestpractice.com
[9]Taran FA, Stewart EA, Brucker S. Adenomyosis: epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe Frauenheilkd. 2013 Sep;73(9):924-31.
https://www.thieme-connect.de/products/ejournals/html/10.1055/s-0033-1350840
http://www.ncbi.nlm.nih.gov/pubmed/24771944?tool=bestpractice.com
[17]Isaacson K, Loring M. Symptoms of adenomyosis and overlapping diseases. Semin Reprod Med. 2020 May;38(2-03):144-50.
http://www.ncbi.nlm.nih.gov/pubmed/33352607?tool=bestpractice.com
[38]Bulun SE, Yildiz S, Adli M, et al. Endometriosis and adenomyosis: shared pathophysiology. Fertil Steril. 2023 May;119(5):746-50.
https://www.fertstert.org/article/S0015-0282(23)00211-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36925057?tool=bestpractice.com
[39]Parazzini F, Vercellini P, Panazza S, et al. Risk factors for adenomyosis. Hum Reprod. 1997 Jun;12(6):1275-9.
http://www.ncbi.nlm.nih.gov/pubmed/9222017?tool=bestpractice.com
For patients with AUB, the FIGO classification system employs the PALM-COEIN mnemonic to categorise potential causes for, or contributors to, a patient's symptoms, dividing them into structural factors (Polyp, Adenomyosis, Leiomyomas, Malignancy) and non-structural factors (Coagulation, Ovulation, Endometrial, Iatrogenic), as well as a category for potential factors not otherwise classified (N).[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
[57]Jain V, Munro MG, Critchley HOD. Contemporary evaluation of women and girls with abnormal uterine bleeding: FIGO Systems 1 and 2. Int J Gynaecol Obstet. 2023 Aug;162 Suppl 2(suppl 2):29-42.
https://obgyn.onlinelibrary.wiley.com/doi/10.1002/ijgo.14946
http://www.ncbi.nlm.nih.gov/pubmed/37538019?tool=bestpractice.com
Adenomyosis is most commonly confirmed histopathologically in women aged 35 years to menopause. In this age group, anovulatory cycles are a common aetiology of AUB (reflecting the normal physiological decline in ovarian function). Other differentials to consider in this age group include endometrial hyperplasia, cancer, endometrial atrophy, or leiomyomas.[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
Consider hysteroscopy and concomitant histopathological analysis via endometrial biopsy to rule out malignancy in patients with AUB who are at high risk of endometrial pathology.[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
See Assessment of abnormal uterine bleeding.
Initial investigations
The definitive method for diagnosing adenomyosis is via histopathological examination.[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
However, in most cases, this only allows for retrospective diagnosis following hysterectomy. TVUS or MRI are now commonly used to make a clinical diagnosis of adenomyosis.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[11]Royal College of Obstetricians and Gynaecologists. The initial management of chronic pelvic pain (Green-top Guideline no. 41). May 2012 [internet publication].
https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-initial-management-of-chronic-pelvic-pain-green-top-guideline-no-41
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
These imaging techniques are non-invasive and provide valuable information about the extent and location of adenomyotic lesions.
Note that there is still a lack of standardised diagnostic criteria for both imaging and histopathological modalities.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
Improved standardisation and training are needed to enhance the accuracy of adenomyosis diagnosis.
Transvaginal ultrasound (TVUS)
Offer TVUS as the first-line investigation in all patients presenting with signs and symptoms suggestive of adenomyosis (in particular, significant dysmenorrhoea and/or menorrhagia and/or a diffusely enlarged, bulky, tender, globular uterus on clinical examination).[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
[51]American College of Radiology. ACR appropriateness criteria: abnormal uterine bleeding. 2020 [internet publication].
https://acsearch.acr.org/docs/69458/Narrative
Ultrasound is widely available and is generally well tolerated.[58]O'Shea A, Figueiredo G, Lee SI. Imaging diagnosis of adenomyosis. Semin Reprod Med. 2020 May;38(2-03):119-28.
http://www.ncbi.nlm.nih.gov/pubmed/33197946?tool=bestpractice.com
Two-dimensional (2D) TVUS is the most commonly used imaging modality for diagnosing adenomyosis. Three-dimensional (3D) TVUS allows for better visualisation of the junctional zone; one systematic review of the effectiveness of various non-surgical methods for the diagnosis of adenomyosis found that combining 2D TVUS with 3D TVUS improved specificity from 64% with 2D TVUS alone to 81% when combined with 3D TVUS, without a significant difference in sensitivity (85% compared with 84%).[59]Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018 Feb;25(2):257-64.
http://www.ncbi.nlm.nih.gov/pubmed/28864044?tool=bestpractice.com
TVUS is preferred over transabdominal ultrasound due to superior imaging capabilities, including higher resolution and better image quality, achieved through the utilisation of higher transducer frequencies and the elimination of beam deformation caused by the ventral abdomen.[60]Moorthy RS. Transvaginal sonography. Med J Armed Forces India. 2000 Jul;56(3):181-3.
https://pmc.ncbi.nlm.nih.gov/articles/PMC5532046
http://www.ncbi.nlm.nih.gov/pubmed/28790701?tool=bestpractice.com
In adolescents, or if an adult patient declines TVUS, a transabdominal ultrasound or MRI may be offered as an alternative.[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
TVUS should be used to assess for direct and indirect features of adenomyosis.[61]Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015 Sep;46(3):284-98.
https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.14806
http://www.ncbi.nlm.nih.gov/pubmed/25652685?tool=bestpractice.com
[62]Harmsen MJ, Van den Bosch T, de Leeuw RA, et al. Consensus on revised definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: results of modified Delphi procedure. Ultrasound Obstet Gynecol. 2022 Jul;60(1):118-31.
https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.24786
http://www.ncbi.nlm.nih.gov/pubmed/34587658?tool=bestpractice.com
Note that the presence of indirect features in the absence of direct features is not conclusive for the presence of adenomyosis.[62]Harmsen MJ, Van den Bosch T, de Leeuw RA, et al. Consensus on revised definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: results of modified Delphi procedure. Ultrasound Obstet Gynecol. 2022 Jul;60(1):118-31.
https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.24786
http://www.ncbi.nlm.nih.gov/pubmed/34587658?tool=bestpractice.com
TVUS may also be used to assess for:[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
Extent of disease, classified as mild (<25% of myometrium), moderate (25% to 50% of myometrium), or severe (>50% of myometrium).
Location of disease (anterior wall, posterior wall, left lateral, right lateral, fundus).
Size of the largest lesion or affected area.
Focal versus diffuse disease.
In focal disease, nodular aggregates of endometrial glands and stroma are surrounded by normal myometrium, whereas in diffuse disease there are endometrial glands and stroma distributed throughout the myometrium.[1]Van den Bosch T, de Bruijn AM, de Leeuw RA, et al. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol. 2019 May;53(5):576-82.
https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.19096
http://www.ncbi.nlm.nih.gov/pubmed/29790217?tool=bestpractice.com
One group has proposed that an adenomyotic lesion should be classified as focal if >25% of the lesion is surrounded by normal myometrium and as diffuse if <25% is surrounded by normal myometrium.[1]Van den Bosch T, de Bruijn AM, de Leeuw RA, et al. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol. 2019 May;53(5):576-82.
https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.19096
http://www.ncbi.nlm.nih.gov/pubmed/29790217?tool=bestpractice.com
If there is difficulty distinguishing focal from diffuse disease, the adenomyosis should be classified as diffuse.[1]Van den Bosch T, de Bruijn AM, de Leeuw RA, et al. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol. 2019 May;53(5):576-82.
https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.19096
http://www.ncbi.nlm.nih.gov/pubmed/29790217?tool=bestpractice.com
Uterine layer involvement (junctional zone, middle myometrium, or outer myometrium).
The limitations of ultrasound as a diagnostic test include the field of view and inter-observer variability, which can affect its generalisability.[58]O'Shea A, Figueiredo G, Lee SI. Imaging diagnosis of adenomyosis. Semin Reprod Med. 2020 May;38(2-03):119-28.
http://www.ncbi.nlm.nih.gov/pubmed/33197946?tool=bestpractice.com
MRI
Do not use MRI as a routine investigation for suspected adenomyosis.[51]American College of Radiology. ACR appropriateness criteria: abnormal uterine bleeding. 2020 [internet publication].
https://acsearch.acr.org/docs/69458/Narrative
MRI may be appropriate, where available:
for surgical or interventional treatment planning[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[50]American College of Obstetricians and Gynecologists; Committee on Practice Bulletins - Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012 Jul;120(1):197-206.
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2012/07/diagnosis-of-abnormal-uterine-bleeding-in-reproductive-aged-women
http://www.ncbi.nlm.nih.gov/pubmed/22914421?tool=bestpractice.com
following TVUS if there is inconclusive sonographic evaluation of adenomyosis or if there is suspicion of significant co-existing pelvic pathology[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
[51]American College of Radiology. ACR appropriateness criteria: abnormal uterine bleeding. 2020 [internet publication].
https://acsearch.acr.org/docs/69458/Narrative
for women who decline TVUS or for whom it is unsuitable (e.g., adolescents).[3]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. Mar 2018; updated May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
MRI has lower false-positive rates and reduced inter-observer variability when compared with TVUS.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
MRI has a similar sensitivity to TVUS (reported range: 77% to 78%), but its specificity is higher (reported range: 88% to 93%).[66]Tellum T, Nygaard S, Lieng M. Noninvasive diagnosis of adenomyosis: a structured review and meta-analysis of diagnostic accuracy in imaging. J Minim Invasive Gynecol. 2020 Feb;27(2):408-18.e3.
http://www.ncbi.nlm.nih.gov/pubmed/31712162?tool=bestpractice.com
[67]Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021 Nov;40(11):2289-306.
http://www.ncbi.nlm.nih.gov/pubmed/33502767?tool=bestpractice.com
[68]Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010 Nov;89(11):1374-84.
https://obgyn.onlinelibrary.wiley.com/doi/10.3109/00016349.2010.512061
http://www.ncbi.nlm.nih.gov/pubmed/20932128?tool=bestpractice.com
The positive likelihood ratio for adenomyosis (the probability that a positive test would be expected in a patient with the condition divided by the probability that a positive test would be expected in a patient without the condition) is higher with MRI compared with TVUS (11.98 vs. 4.93, respectively).[67]Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021 Nov;40(11):2289-306.
http://www.ncbi.nlm.nih.gov/pubmed/33502767?tool=bestpractice.com
MRI is better able than TVUS to differentiate between adenomyosis and leiomyomas (uterine fibroids).[49]Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408.
http://www.ncbi.nlm.nih.gov/pubmed/30198563?tool=bestpractice.com
[68]Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010 Nov;89(11):1374-84.
https://obgyn.onlinelibrary.wiley.com/doi/10.3109/00016349.2010.512061
http://www.ncbi.nlm.nih.gov/pubmed/20932128?tool=bestpractice.com
MRI can be used to diagnose adenomyosis by evaluating the thickness of the junctional zone, which appears as a distinct T2 hypointense band separating the T2-hyperintense endometrium and the intermediate-intensity myometrium.[69]Novellas S, Chassang M, Delotte J, et al. MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. AJR Am J Roentgenol. 2011 May;196(5):1206-13.
https://www.ajronline.org/doi/10.2214/AJR.10.4877
http://www.ncbi.nlm.nih.gov/pubmed/21512093?tool=bestpractice.com
T2 hypointense junctional zone thickness >12 mm is indicative of adenomyosis (with a sensitivity of 72% and specificity of 86%), while a thickness <8 mm excludes the disease.[70]Tamai K, Togashi K, Ito T, et al. MR imaging findings of adenomyosis: correlation with histopathologic features and diagnostic pitfalls. Radiographics. 2005 Jan-Feb;25(1):21-40.
http://www.ncbi.nlm.nih.gov/pubmed/15653584?tool=bestpractice.com
T2 hyperintense foci can be observed, representing cystic dilation of the endometrial glands. These are similar to anechoic myometrial cysts seen on TVUS.[63]Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001 Nov;16(11):2427-33.
http://www.ncbi.nlm.nih.gov/pubmed/11679533?tool=bestpractice.com
[71]Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017 Dec;8(6):549-56.
https://link.springer.com/article/10.1007/s13244-017-0576-z
http://www.ncbi.nlm.nih.gov/pubmed/28980163?tool=bestpractice.com
T1 signal hyperintensity can be seen if haemorrhage occurs in these foci. This is highly specific (up to 95%) for adenomyosis.[63]Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001 Nov;16(11):2427-33.
http://www.ncbi.nlm.nih.gov/pubmed/11679533?tool=bestpractice.com
[71]Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017 Dec;8(6):549-56.
https://link.springer.com/article/10.1007/s13244-017-0576-z
http://www.ncbi.nlm.nih.gov/pubmed/28980163?tool=bestpractice.com
Direct features of adenomyosis that may be seen on MRI include:
myometrial cysts (sensitivity of 60% and specificity of 96%)[72]Takeuchi M, Matsuzaki K. Adenomyosis: usual and unusual imaging manifestations, pitfalls, and problem-solving MR imaging techniques. Radiographics. 2011 Jan-Feb;31(1):99-115.
http://www.ncbi.nlm.nih.gov/pubmed/21257936?tool=bestpractice.com
adenomyoma (a myometrial mass that does not involve the uterine serosa or junctional zone)[73]Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018 Mar;109(3):389-97.
https://www.fertstert.org/article/S0015-0282(18)30024-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29566851?tool=bestpractice.com
external adenomyosis (involvement of the serosa but not the junctional zone).[73]Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018 Mar;109(3):389-97.
https://www.fertstert.org/article/S0015-0282(18)30024-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29566851?tool=bestpractice.com
Diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) images are beneficial.[58]O'Shea A, Figueiredo G, Lee SI. Imaging diagnosis of adenomyosis. Semin Reprod Med. 2020 May;38(2-03):119-28.
http://www.ncbi.nlm.nih.gov/pubmed/33197946?tool=bestpractice.com
DWI can be helpful in differentiating between benign and malignant neoplasms when characterising lesions.[74]Davarpanah AH, Kambadakone A, Holalkere NS, et al. Diffusion MRI of uterine and ovarian masses: identifying the benign lesions. Abdom Radiol (NY). 2016 Dec;41(12):2466-75.
http://www.ncbi.nlm.nih.gov/pubmed/27660280?tool=bestpractice.com
[75]McDermott S, Oei TN, Iyer VR, et al. MR imaging of malignancies arising in endometriomas and extraovarian endometriosis. Radiographics. 2012 May-Jun;32(3):845-63.
http://www.ncbi.nlm.nih.gov/pubmed/22582363?tool=bestpractice.com
DCE is useful to distinguish endometrial pathology, such as polyps and tumours, from myometrial lesions such as fibroids, as myometrial lesions are typically hypovascular.[76]Jha RC, Zanello PA, Ascher SM, et al. Diffusion-weighted imaging (DWI) of adenomyosis and fibroids of the uterus. Abdom Imaging. 2014 Jun;39(3):562-9.
http://www.ncbi.nlm.nih.gov/pubmed/24531353?tool=bestpractice.com
Note that MRI should not be performed during menstruation or the early proliferative phase to avoid pseudo-widening of the junctional zone, which can occur due to decreased signal intensity of the myometrium.[72]Takeuchi M, Matsuzaki K. Adenomyosis: usual and unusual imaging manifestations, pitfalls, and problem-solving MR imaging techniques. Radiographics. 2011 Jan-Feb;31(1):99-115.
http://www.ncbi.nlm.nih.gov/pubmed/21257936?tool=bestpractice.com
There is limited research associating specific features of adenomyosis on MRI with clinical symptoms and symptom severity.[77]Rees CO, Nederend J, Mischi M, et al. Objective measures of adenomyosis on MRI and their diagnostic accuracy - a systematic review & meta-analysis. Acta Obstet Gynecol Scand. 2021 Aug;100(8):1377-91.
https://obgyn.onlinelibrary.wiley.com/doi/10.1111/aogs.14139
http://www.ncbi.nlm.nih.gov/pubmed/33682087?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Sagittal MRI of a woman's pelvis showing a uterus with adenomyosis in the posterior wall. Gross enlargement of the posterior wall is noted, with many foci of hyperintensityCase courtesy of Dr Varun Babu, Radiopaedia.org. From the case rID: 43504; reproduced under the Creative Commons CC BY-SA 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/ ) [Citation ends].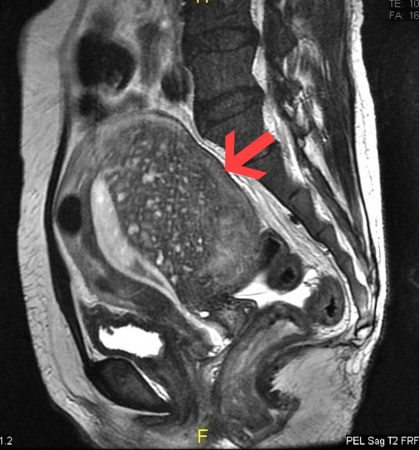
Other investigations
Histological diagnosis of adenomyosis following hysterectomy
Histopathological diagnosis (usually following hysterectomy) is considered the definitive method for confirming adenomyosis; this involves identifying the presence of endometrial stroma and glandular tissue within the smooth muscle of the myometrium.[78]Gunther R, Walker C. Adenomyosis. In: National Library of Medicine: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025.
https://www.ncbi.nlm.nih.gov/books/NBK539868
http://www.ncbi.nlm.nih.gov/pubmed/30969690?tool=bestpractice.com
This can provide a definitive diagnosis in women who have no wish for future fertility and in whom hysterectomy is considered the most appropriate option for managing adenomyosis.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
In women who desire future fertility, or when hysterectomy is not feasible or not acceptable to the patient, imaging remains the most appropriate modality to make a clinical diagnosis.[16]Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A) - pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017 Apr;40:68-81.
http://www.ncbi.nlm.nih.gov/pubmed/27810281?tool=bestpractice.com
Note that there are no universally accepted histological criteria for diagnosing adenomyosis, and the accuracy of diagnosis can be influenced by several factors including:[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
Variability in the depth of myometrial invasion, which plays a crucial role in categorising the pathology as adenomyosis.[16]Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A) - pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017 Apr;40:68-81.
http://www.ncbi.nlm.nih.gov/pubmed/27810281?tool=bestpractice.com
Extent of uterine sectioning during tissue sampling.[79]Munro MG. Classification and reporting systems for adenomyosis. J Minim Invasive Gynecol. 2020 Feb;27(2):296-308.
http://www.ncbi.nlm.nih.gov/pubmed/31785418?tool=bestpractice.com
Inter-observer variability.[79]Munro MG. Classification and reporting systems for adenomyosis. J Minim Invasive Gynecol. 2020 Feb;27(2):296-308.
http://www.ncbi.nlm.nih.gov/pubmed/31785418?tool=bestpractice.com
Hysterosalpingography (HSG)
Note that although HSG is not typically used to diagnose adenomyosis, it is often performed on patients with infertility who may also have adenomyosis; as a result, adenomyosis may be incidentally detected during an HSG examination.[80]Simpson WL Jr, Beitia LG, Mester J. Hysterosalpingography: a reemerging study. Radiographics. 2006 Mar-Apr;26(2):419-31.
http://www.ncbi.nlm.nih.gov/pubmed/16549607?tool=bestpractice.com
Adenomyosis is characterised by heterotopic endometrial foci that extend into the myometrium, which also fills with contrast. As a result, patients with adenomyosis often exhibit a distinct appearance of contrast outpouching into the myometrium at the margins of the endometrial cavity.[80]Simpson WL Jr, Beitia LG, Mester J. Hysterosalpingography: a reemerging study. Radiographics. 2006 Mar-Apr;26(2):419-31.
http://www.ncbi.nlm.nih.gov/pubmed/16549607?tool=bestpractice.com
Tissue-sampling techniques
Fertility-sparing tissue-sampling techniques are under investigation for their potential to obtain diagnostic samples while preserving reproductive potential.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
Directed tissue-sampling techniques are reserved for specific scenarios in which confirmatory diagnosis may be of benefit.[2]Dason ES, Maxim M, Sanders A, et al; Society of Obstetricians and Gynaecologists of Canada (SOGC). Guideline no. 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023 Jun;45(6):417-29.e1.
https://www.jogc.com/article/S1701-2163(23)00307-9/abstract
http://www.ncbi.nlm.nih.gov/pubmed/37244746?tool=bestpractice.com
If laparoscopy is performed for other fertility-sparing reasons and there is a preliminary suspicion of adenomyosis, such biopsy techniques may have a role in providing a confirmatory diagnosis and guiding future surgical treatment recommendations for these patients.[81]Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020 Feb;27(2):344-51.
http://www.ncbi.nlm.nih.gov/pubmed/31499191?tool=bestpractice.com
These techniques can be performed either through intrauterine tissue sampling using a hysteroscopic approach or via extrauterine sampling, where a myometrial biopsy is obtained through ultrasound or laparoscopy guidance.[81]Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020 Feb;27(2):344-51.
http://www.ncbi.nlm.nih.gov/pubmed/31499191?tool=bestpractice.com
The sensitivity of these tissue-sampling techniques varies significantly, ranging from 22.3% to 97.8%, with the highest sensitivity being reported for laparoscopic-guided uterine biopsy with a 14-gauge needle.[82]Jeng CJ, Huang SH, Shen J, et al. Laparoscopy-guided myometrial biopsy in the definite diagnosis of diffuse adenomyosis. Hum Reprod. 2007 Jul;22(7):2016-9.
http://www.ncbi.nlm.nih.gov/pubmed/17428879?tool=bestpractice.com
The variation in sensitivity may be influenced by factors such as the number and location of biopsies, as well as the technique employed for biopsy collection.[81]Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020 Feb;27(2):344-51.
http://www.ncbi.nlm.nih.gov/pubmed/31499191?tool=bestpractice.com
The specificity of these techniques is generally higher, ranging from 78.5% to 100%.[81]Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020 Feb;27(2):344-51.
http://www.ncbi.nlm.nih.gov/pubmed/31499191?tool=bestpractice.com
