Recommendations
Urgent
Assess for signs of haemodynamic instability (ABCDE approach), which can indicate a high-risk (massive/central) PE.
Seek senior support and escalate to critical care if suspected.[67][74][76]
If the patient is in peri-arrest/cardiac arrest and PE is suspected it is common practice to give thrombolysis immediately without waiting for results of investigations (based on discussion between senior clinicians).
Get an urgent senior review if systolic blood pressure (SBP) is <90 mmHg and the jugular venous pressure (JVP) is elevated to determine whether intravenous fluids need to be given. Give intravenous fluids if SBP is <90 mmHg and the JVP is not elevated.[67]
Give either normal saline or Hartmann’s; give a cautious fluid challenge of ≤500 mL over 15-30 minutes.[67]
Monitor for signs of heart failure.[67] The leading cause of death in patients with high-risk PE is acute right ventricular (RV) failure with resulting hypotension.
Ensure early respiratory support.
Start oxygen if saturations are <90%.[67]
Titrate oxygen to target saturations of 94% to 98% (or 88% to 92% for patients at risk of hypercapnic failure).[67]
Use high-flow oxygen and mechanical ventilation if the patient is extremely unstable (e.g., cardiac arrest). Beware that this can worsen hypotension so monitor blood pressure closely.[67][150]
Arrange urgent primary reperfusion for any haemodynamically unstable patient (high-risk PE).[67][74]
Systemic thrombolysis is the standard treatment of choice. Currently there is a lack of evidence to support widespread adoption of catheter-based interventional therapies.[151]
Do not allow supportive therapy to delay thrombolysis, which may quickly restore haemodynamic stability.
Use vasoactive drugs concurrently with, or while waiting for, thrombolysis if the patient remains haemodynamically unstable after adequate fluid resuscitation.
Give anticoagulation early if indicated.[67][74][76]
Check the patient has no contraindications to anticoagulation.
You do not need to wait for the results of investigations to give anticoagulation if PE is highly suspected.
Be aware of special cases such as pregnancy, abnormal kidney function, and active cancer as these groups will need a specific type/dose of anticoagulant.
Key Recommendations
[Figure caption and citation for the preceding image starts]: Summary of PE management by risk categoryby BMJ Knowledge Centre [Citation ends].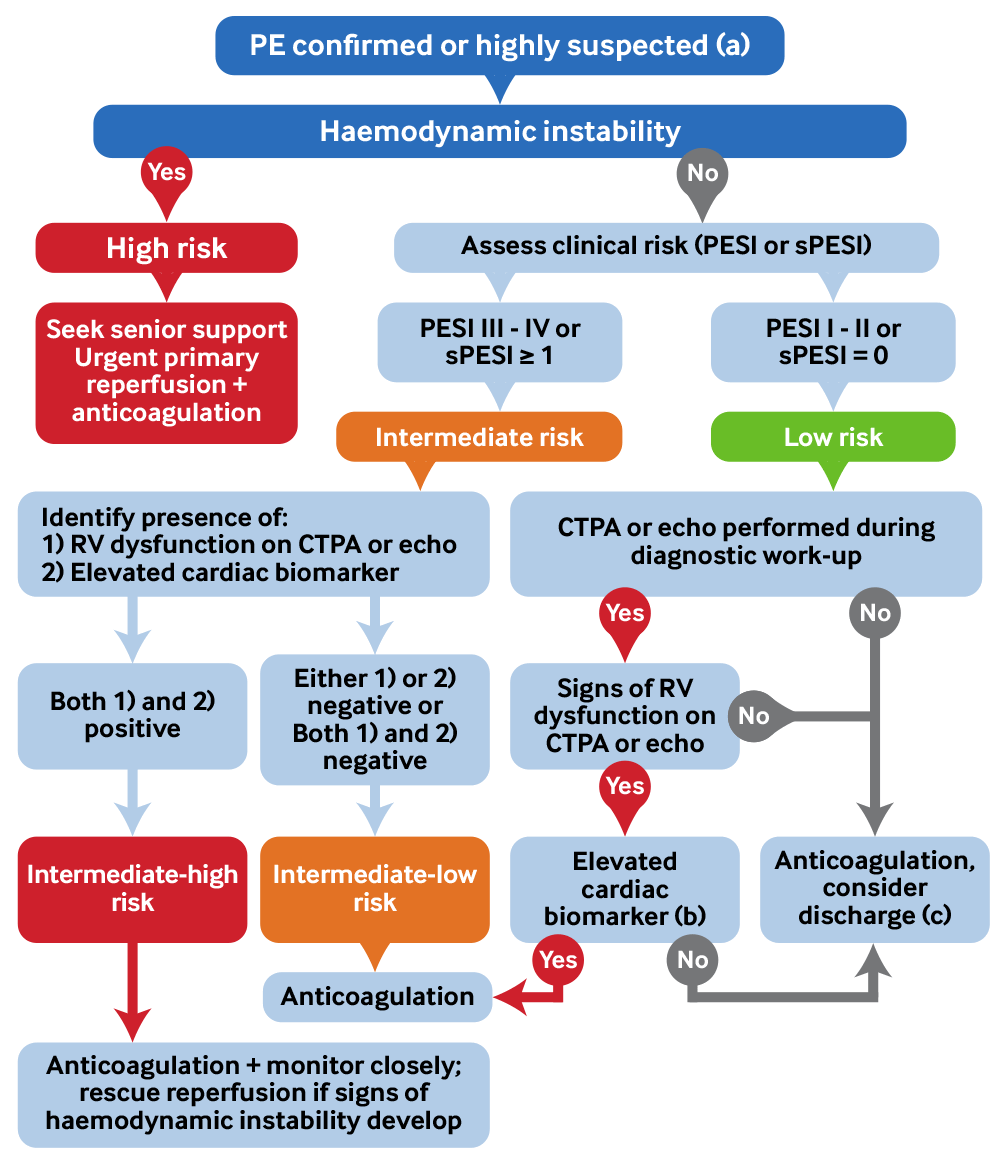
Initial supportive treatment for haemodynamically unstable (high-risk) PE[67][150]
Ensure early respiratory support. Start oxygen if saturations are <90%.[67]
Titrate oxygen to target saturations of 94% to 98% (or 88% to 92% for patients at risk of hypercapnic failure).[67]
Use high-flow oxygen and mechanical ventilation if the patient is extremely unstable (e.g., cardiac arrest) but monitor blood pressure closely.
If the patient is in peri-arrest or cardiac arrest and PE is suspected it is common practice to give thrombolysis without waiting for results of investigations.
This decision will be made by senior clinicians.
Get an urgent senior review if SBP is <90 mmHg and the JVP is elevated to determine whether intravenous fluids need to be given. Give intravenous fluids if SBP is <90 mmHg and the JVP is not elevated.
Give a cautious fluid challenge of ≤500 mL over 15-30 minutes.[67]
Some studies indicate that aggressive volume expansion is of no benefit and may even worsen RV function so monitor for signs of heart failure.[67]
Do not allow supportive therapy to delay thrombolysis in high-risk PE (as long as there are no contraindications) as this may quickly restore haemodynamic stability.
Further acute management of haemodynamically unstable (high-risk) patients[67][74]
Seek senior support when managing any patient who is haemodynamically unstable and has had PE either:
Confirmed on computed tomographic pulmonary angiography (CTPA), OR
Highly suspected (unequivocal signs of RV dysfunction on echocardiography).
Ensure all such patients receive both anticoagulation and thrombolysis, unless contraindicated, as follows:
Anticoagulation with unfractionated heparin (UFH) initially. This should be started prior to primary reperfusion.
Continue anticoagulation with UFH for several hours after the end of thrombolysis before switching to apixaban or rivaroxaban; low molecular weight heparin (LMWH) is an alternative if these are unsuitable.
UFH may be administered during continuous infusion of alteplase, but should be discontinued during infusion of streptokinase or urokinase.[67]
AND
Thrombolysis
Select an accelerated thrombolysis regimen, administered over 2 hours.
In practice, almost any contraindication should be considered only relative in haemodynamically unstable patients with PE because the high mortality risk is likely to outweigh any bleeding risk.
If the patient continues to deteriorate despite thrombolysis (or if thrombolysis is absolutely contraindicated after discussion with haematology), consider surgical embolectomy or percutaneous catheter-directed therapy (depending on local expertise and availability).[151][152]
Give a vasoactive drug concurrently with, or while waiting for, thrombolysis if the patient remains haemodynamically unstable despite adequate intravenous fluids.[67]
Acute-phase anticoagulation in haemodynamically stable patients
Give anticoagulation to all of the following groups unless contraindicated:[67][76]
Patients with confirmed PE either on CTPA or echocardiography
Patients who present with suspected PE and have either a positive D-dimer or a Wells score >4. If PE is subsequently excluded, anticoagulation should be discontinued.
Decide on the type of anticoagulant based on a patient’s comorbidities and contraindications as well as taking into account local guidelines.
Apixaban, rivaroxaban, or LMWH are all options. Dabigatran and edoxaban are not recommended as the initial anticoagulant in these patients because they require lead-in therapy with a parenteral anticoagulant first.
Be aware of special patient groups such as pregnancy, abnormal kidney function, and active cancer as these groups will need a specific type/dose of anticoagulant.
Consult a haematologist if a patient has a contraindication to anticoagulation. Many of these patients, even those with apparent absolute contraindications, may still be able to have a different or altered dose of an anticoagulant.
Risk stratification
Risk stratify haemodynamically stable patients using the Pulmonary Embolism Severity Index (PESI) score or the simplified Pulmonary Embolism Severity Index (sPESI) score to determine which patients may be safely discharged and which need admission with closer monitoring.[67][76][77][106]
Risk stratify intermediate-risk patients (PESI III-IV or sPESI ≥1) further by measuring both their RV function (on CTPA or echocardiography) and a cardiac biomarker.[67]
Patients are classed as intermediate-high risk if:[67]
There are signs of RV dysfunction
AND
The cardiac biomarker is elevated.
Start anticoagulation and monitor intermediate-high risk patients very closely as they are at risk of deterioration. If they become haemodynamically unstable, they will need reperfusion therapy (usually rescue thrombolysis).[67]
Patients are classified as intermediate-low risk (and should be admitted but do not require close monitoring) if there are:[67]
No signs of RV dysfunction AND the cardiac biomarker is normal
OR
Either signs of RV dysfunction OR the cardiac biomarker is elevated.
Consider low-risk patients (PESI I or II, or sPESI 0) for early discharge.
PE does not need to be confirmed on CTPA prior to discharge but it should be performed within 24 hours (if available).[106]
Assessment of RV size/function on CTPA or echocardiography is not obligatory for the identification of low-risk patients for outpatient management.[106]
Consider measuring cardiac biomarkers (high-sensitivity troponin, B-type natriuretic peptide [BNP], or N-terminal pro-B-type natriuretic peptide [NT-proBNP]) in patients who have evidence of RV dysfunction on CTPA/echo if performed during diagnostic work-up and who are suitable for outpatient management. If these are normal they can be considered for discharge. If they are elevated they should be admitted for observation.[106]
Do not use risk stratification scores for haemodynamically unstable patients.
Treat any such patient as high-risk.
Do not use PESI/sPESI scoring in pregnant women or patients with active cancer.
Use HESTIA to risk stratify patients with active cancer.[106]
Ongoing anticoagulation post-PE
The standard duration of anticoagulation should be at least 3 months.[67][76]
Longer-term, or sometimes indefinite, anticoagulation may be needed for secondary prevention after weighing the individual patient’s risk of recurrence versus bleeding risk.
In general, continue the anticoagulant that was started in the acute phase if this is well tolerated.[76]
Anticoagulation can usually be stopped after 3 months following a provoked PE.[67][76]
A provoked PE is one associated with a reversible or transient risk factor that was present in the prior 3 months (e.g., surgery, trauma, immobility, pregnancy, use of oral contraception, or hormone replacement therapy).
Anticoagulation is typically continued for >3 months after an unprovoked PE.[67][76]
The main treatment goals are to:
Provide supportive treatment and give early reperfusion therapy to any patient who is haemodynamically unstable (usually thrombolysis, but in rare cases may be surgical embolectomy/percutaneous catheter-directed therapy).
Ensure anticoagulation is given early to any patient with either confirmed or highly clinically suspected PE.
Risk stratify haemodynamically stable patients to plan ongoing management.
Identify underlying risk factors that have provoked the PE.
Patients with suspected or confirmed PE who present with shock or hypotension are at high risk of in-hospital death, particularly in the first few hours after presentation.[67]
Seek immediate senior input and escalate to the critical care team.
Practical tip
Do not allow supportive therapy to delay thrombolysis in these patients (as long as there are no contraindications) - thrombolysis may quickly restore haemodynamic stability.
It is common practice to give thrombolysis as first-line treatment for any patient who is in peri-arrest/cardiac arrest based on clinical suspicion of PE, without waiting for results from investigations.
In these situations the decision to give thrombolysis would be based on discussion between senior clinicians.
Evidence: High-risk PE
Patients with high-risk PE are at significant risk of death.
The 30-day mortality rate of patients with haemodynamically unstable (high-risk) PE ranges from 16% to 25%, and that of patients with cardiac arrest due to PE ranges from 52% to 65%.[153][154]
Most deaths in patients presenting with shock occur within the first hour after presentation, so rapid therapeutic action is essential to save lives.[7]
The International Cooperative Pulmonary Embolism Registry (ICOPER) showed 90-day mortality rates of 58.3% in patients with high-risk (massive) PE.[21]
The vast majority of patients with PE present without cardiogenic shock and with normal or only mildly reduced blood pressure.
These patients have an overall 30-day mortality rate of about 6% and are considered as non-high-risk patients.[155]
In these patients, right heart dysfunction on echocardiography or evidence of myocardial injury has been shown to be associated with an increased risk of clinical deterioration and/or short-term death. Other factors, such as an elevated troponin (indicating cardiac ischaemia), altered mental status, and the presence of comorbidities, have also been correlated with early clinical deterioration and higher risk of short-term death.[107][156]
In a meta-analysis of six studies involving 2874 haemodynamically stable patients with PE and evidence of myocardial injury and/or right ventricular (RV) dysfunction, follow-up at 30 days showed PE-related death in 90 patients, haemodynamic collapse in 122 patients, and non-fatal symptomatic recurrent PE in 18 patients.[155]
An analysis of 298 consecutive patients with low-risk PE (haemodynamically stable, no rise in cardiac markers, and no evidence of RV dysfunction) found that around two-thirds suffered no clinical deterioration.[157]
Oxygen
Give high-concentration oxygen if saturations are <90%, targeting a saturation of 94% to 98% (or 88% to 92% in any patient at risk of hypercapnic respiratory failure).[67][150]
Assess oxygen saturations early. Hypoxaemia is a typical finding in PE.[67] The patient may be hypoxic at rest if a high-risk (massive) PE is present or only on exertion with a smaller PE.
Use high-flow oxygen and mechanical ventilation if the patient is extremely unstable (e.g., cardiac arrest). Beware that this can worsen haemodynamic instability in a high-risk (massive) PE. Mechanical ventilation induces positive thoracic pressure, which may reduce venous return and worsen right ventricular (RV) failure.[67]
How to insert a tracheal tube in an adult using a laryngoscope.
Fluid resuscitation
Get an urgent senior review if systolic blood pressure (SBP) is <90 mmHg and the jugular venous pressure (JVP) is elevated to determine whether intravenous fluids need to be given.[67] Give intravenous fluids if SBP is <90 mmHg and the JVP is not elevated.[67]
Give intravenous fluids with caution and start with a fluid challenge of ≤500 mL over 15-30 minutes.[67] Determine further fluid based on clinical response.[67]
Use normal saline (0.9% NaCl) or Hartmann’s/Ringer’s lactate.[161] Guidelines differ in their recommendations on choice of fluid and clinical practice varies widely so check local protocols.
Monitor closely for signs of right heart failure.[67]
The leading cause of death in patients with high-risk (massive) PE is acute RV failure with resulting hypotension.[67]
Patients with haemodynamically unstable PE may require early intravenous fluid resuscitation but should be monitored for signs of right heart failure.
Evidence: Volume expansion in PE
Aggressive volume expansion in patients with haemodynamically unstable PE is of no benefit and may even worsen RV function.
There is only very scarce and conflicting evidence on the balance of risks versus benefits from fluid resuscitation in patients with PE who are haemodynamically unstable.
One prospective study of 13 patients with high-risk haemodynamically unstable PE suggested that fluid loading (an infusion of 500 mL of dextran 40 over 20 minutes) can improve haemodynamic status. It was shown to increase RV preload, which was associated with an increase in cardiac index without reducing the RV ejection fraction.[162]
In contrast, earlier experimental studies in dogs indicated that aggressive volume expansion may be of no benefit and may even worsen RV function. They found that when pulmonary vascular resistance was normal, volume expansion increased stroke volume and mean blood pressure whereas when RV afterload was increased, this volume expansion resulted in RV failure.[163][164]
The 2019 European Society of Cardiology guideline states that evidence to date indicates that aggressive volume expansion is of no benefit and may even worsen RV function.[67]
Give anticoagulation to all of the following patients (unless contraindicated):[67][74][76]
Those with PE confirmed either on computed tomographic pulmonary angiography (CTPA) or echocardiography
Those who present with high clinical probability of PE, based on either a positive D-dimer or a Wells score >4. If PE is subsequently excluded, anticoagulation should be discontinued.
Practical tip
In patients who are haemodynamically unstable, evidence of right ventricular dysfunction on echocardiography is sufficient to warrant urgent anticoagulation and thrombolysis.
These patients are normally too unstable to undergo CTPA.
The management of subsegmental PE remains controversial.
Practice varies but surveillance rather than anticoagulation is considered the best option for most patients.[67]
Evidence: Subsegmental PE
Evidence suggests subsegmental PE may be of little clinical significance in most cases.
The decision over whether to anticoagulate subsegmental PE (SSPE) remains controversial and practice varies widely. Seek specialist advice.
Increasing use of CTPA has increased the number of patients diagnosed with SSPE without any change in mortality rates, suggesting these PEs may not be clinically relevant.[123][124][125][126]
There may also be overdiagnosis of SSPE. Evidence shows low clinical agreement between radiologists when diagnosing patients with small distal clots as they are subtle and hard to distinguish from artefact.[165]
European and American guidelines suggest weighing up clinical probability against the bleeding risk but recommend surveillance rather than anticoagulation for patients with SSPE who have no proximal deep vein thrombosis (DVT) and have a low risk of recurrent venous thromboembolism (VTE).[67][166]
Evidence on outcomes in patients who are diagnosed with SSPE is scarce and is based on small numbers of patients, making it difficult to formulate evidence-based guidance.
Some studies have shown that anticoagulation may be of no clinical benefit. One meta-analysis examined 750 patients with SSPE, of whom 81% were treated with anticoagulation. Over 90 days there was no difference in mortality or recurrence of PE between those who did and did not receive anticoagulation but 8% of treated patients had an episode of bleeding.[167] In an observational study, 15% (82 patients) of PEs found on CTPA were SSPEs. Around half of these patients were anticoagulated, and two developed life-threatening bleeding. No patient had an identified recurrent PE, whether or not they were anticoagulated.[168] The risk of recurrence of VTE in patients with SSPE without a concurrent DVT has been found to be insignificant in retrospective studies.[126][169]
On the other hand, a retrospective study of over 3000 patients showed that the rate of recurrence of PE during anticoagulant therapy was the same in patients with SSPE and those with larger PE (i.e., segmental or lobar). It also showed that VTE recurrence was higher with SSPE than in those in whom PE was excluded.[170]
Contraindications to anticoagulation
Consult a haematologist if a patient has a contraindication to anticoagulation.
Many patients with relative contraindications will still be able to have a different choice or altered dose of anticoagulation, but a specialist opinion is needed to weigh up the benefit-risk balance.
Absolute contraindications are rare but include:[171]
Active bleeding
Recent intracranial haemorrhage
Recent, planned, or emergent surgery or procedure with high bleeding ris
Platelet count <50,000/uL
Severe bleeding diathesis.
Relative contraindications include:[171]
Recurrent but inactive gastrointestinal bleeding
Intracranial or spinal tumour
Recent, planned, or emergent surgery or procedure with intermediate bleeding risk
Major trauma including cardiopulmonary resuscitation
Aortic dissection
Platelet count <150,000/uL.
Remember too that each anticoagulant may have its own specific relative and absolute contraindications (e.g., heparin is contraindicated in patients with a history of heparin-induced thrombocytopenia) and these should be checked before starting treatment.
Practical tip
Peptic ulcer disease with no history of bleeding or faecal occult blood is not a contraindication to anticoagulation.[171]
Anticoagulation is safe in most trauma and neurosurgical patients after the first or second postoperative week and in most stroke patients without haemorrhage.[171]
Patients with spinal cord injury without haematomyelia may still be considered for anticoagulation.[171]
Types of anticoagulation
Base your choice of anticoagulant on the patient’s comorbidities and contraindications as well as taking account of local guidelines.[76]
Acute-phase anticoagulation
Haemodynamically unstable (high-risk) PE
For patients with PE and haemodynamic instability, use unfractionated heparin (UFH).[67]
UFH has a short half-life, is easy to monitor, and is readily reversed by protamine.[67]
Start anticoagulation with UFH prior to primary reperfusion with thrombolysis (or in rare cases surgical embolectomy/catheter-directed therapy).
Continue anticoagulation with UFH for several hours after the end of thrombolysis before switching to apixaban or rivaroxaban; low molecular weight heparin (LMWH) is an alternative if these are not suitable. UFH may be administered during continuous infusion of alteplase, but should be discontinued during infusion of streptokinase or urokinase.[67] In the UK it is common practice to stop UFH within 24 hours.
If switching to rivaroxaban or apixaban, these drugs may be started after stopping UFH without the need for lead-in therapy with a parenteral anticoagulant.
Acute-phase treatment consists of an increased dose of the oral anticoagulant over the first 3 weeks (for rivaroxaban), or over the first 7 days (for apixaban).
If switching to LMWH, the total duration of treatment with UFH and then LMWH should be at least 5 days.[67][76]
If ongoing anticoagulation will be with edoxaban or dabigatran, at least 5 days of lead-in therapy with a parenteral anticoagulant is required first. Stop the parenteral anticoagulant before starting dabigatran or edoxaban.[106]
OR
If ongoing anticoagulation will be with warfarin, ensure overlap with a parenteral anticoagulant for at least 5 days or until the INR is ≥2 for at least two consecutive readings (whichever is the longer), followed by a vitamin K antagonist (VKA) on its own.[76] Seek specialist advice to decide when to start warfarin.
Practical tip
If the patient weighs <50 kg or >120 kg consider using an anticoagulant with monitoring of therapeutic levels (e.g., warfarin).[76]
Seek specialist advice when switching between anticoagulants.
The protocol depends on which anticoagulant you are switching to and from. Haematology input is important.
Be aware of special patient groups such as pregnancy, abnormal kidney function, and active cancer as these groups will need a specific type/dose of anticoagulant. If the patient has triple positive antiphospholipid syndrome, seek advice from a haematologist.
Evidence: LMWH in high-risk PE
There is insufficient evidence to support use of LMWH in haemodynamically unstable (high-risk) PE.
There is some evidence that LMWH has a similar safety profile to UFH when used prior to thrombolysis.
A prospective, observational multicentre trial showed similar bleeding rates following thrombolysis in patients who had UFH or LMWH. However this evidence remains limited.
There is not enough evidence to recommend LMWH as a first-line option for patients with PE who are haemodynamically unstable.[172]
Haemodynamically stable PE (with the exception of special cases)
Start initial anticoagulation as soon as possible with either apixaban or rivaroxaban; LMWH is an alternative if these are not suitable. Check local protocols.[76]
If using rivaroxaban or apixaban, these drugs may be started without the need for lead-in therapy with LMWH first.
Acute-phase treatment consists of an increased dose of the oral anticoagulant over the first 3 weeks (for rivaroxaban), or over the first 7 days (for apixaban).
The 2018 British Thoracic Society guideline recommends using a single direct-acting oral anticoagulant (DOAC) in patients being considered for outpatient management to minimise potential confusion over dosing and administration.[106]
If using LMWH, continue treatment for at least 5 days.[67][76]
If ongoing anticoagulation will be with edoxaban or dabigatran, at least 5 days of lead-in therapy with a parenteral anticoagulant is required first.
If ongoing anticoagulation will be with warfarin, start warfarin within 24 hours of diagnosis and ensure overlap with LMWH for at least 5 days or until the INR is ≥2 for at least two consecutive readings (whichever is the longer), followed by a VKA on its own.[76]
Practical tip
If the patient weighs <50 kg or >120 kg consider using an anticoagulant with monitoring of therapeutic levels (e.g., warfarin).[76]
DOACs have non-inferior efficacy and are possibly safer, particularly in terms of major bleeding, than the standard regimen of LMWH plus warfarin.[67][106][173][174][175][176]
Select a DOAC after discussion with the patient about which regimen would be most suited to them, as well as taking into account the risks and benefits of each DOAC.
Practical tip
Never give a DOAC simultaneously with parenteral anticoagulation.
While warfarin is started at the same time as a parenteral anticoagulant and overlapped for at least 5 days or until the INR is ≥2 for at least two consecutive readings (whichever is the longer), followed by a VKA on its own, DOACs should never be overlapped or given at the same time as a parenteral anticoagulant.
Apixaban and rivaroxaban may be started without the need for lead-in therapy with a parenteral anticoagulant first. Either of these DOACs can be used as a single-drug approach; this is why BTS guidelines recommend them as the preferred DOAC options in any patient who might be suitable for early discharge.[106]
However, dabigatran and edoxaban require at least 5 days lead-in therapy with a parenteral anticoagulant before starting treatment. The parenteral anticoagulant should be stopped before dabigatran or edoxaban are started.
Evidence: DOACs versus heparin/warfarin
DOACs have emerged as an equally effective and safer option than heparin/warfarin.
There is now strong evidence that DOACs are non-inferior and have a favourable safety profile for management of PE when compared with either LMWH/warfarin or fondaparinux.[67][106]
Results of the major trials using DOACs in the treatment of VTE indicate that these agents are non-inferior (in terms of efficacy) and possibly safer (particularly in terms of major bleeding) than the standard heparin/warfarin regimen.[67][173][174][175][176][177][178][179] [
 ]
[Evidence B]
]
[Evidence B]A Cochrane review concluded there was:
No difference between dabigatran and the standard regimen in preventing recurrent PE (odds ratio [OR] 1.02, 95% CI 0.50 to 2.04), recurrent VTE (OR 0.93, 95% CI 0.52 to 1.66), and DVT (OR 0.79, 95% CI 0.29 to 2.13), or in causing major bleeding (OR 0.50, 95% CI 0.15 to 1.68).
No difference between the factor Xa inhibitors (apixaban, rivaroxaban, edoxaban) compared with the standard regimen, in preventing recurrent VTE (OR 0.85, 95% CI 0.15 to 1.68), DVT (OR 0.72, 95% CI 0.39 to 1.32), and all-cause mortality (OR 1.16, 95% CI 0.59 to 1.62), or in causing major bleeding (OR 0.97, 95% CI 0.59 to 1.62).
High time in therapeutic range values were achieved under warfarin treatment in all trials. On the other hand, the study populations included relatively young patients, very few of whom had cancer.
Evidence: DOAC choice
There is a lack of evidence comparing different DOACs in PE.
There are no trials that have directly compared different DOACs with each other, so it is difficult to determine which are the most effective for PE.
However, a systematic review, network meta-analysis, and cost effectiveness analysis has indirectly compared DOACs with each other for prevention of stroke in patients with atrial fibrillation.[180]
The authors concluded that apixaban 5 mg twice daily has the highest expected incremental net benefit, followed by rivaroxaban 20 mg once daily, edoxaban 60 mg once daily, and dabigatran 150 mg twice daily. It should be emphasised that this analysis was looking at evidence relating to patients with atrial fibrillation on longer-term anticoagulation for stroke prevention. However the different risks/benefits of each anticoagulant can be taken into account when deciding which DOAC to use for a patient with PE.[180]
Reversal agents exist for dabigatran, apixaban, and rivaroxaban if patients develop serious bleeding or require surgery. There is no licensed reversal agent yet for edoxaban.
Idarucizumab is licensed to reverse the effect of dabigatran.
Results from an ongoing, uncontrolled, phase III, cohort study (RE‑VERSE AD) of 90 adults taking dabigatran who had either serious bleeding or required urgent surgery, showed that treatment with idarucizumab reversed the anticoagulant effect of dabigatran (median maximum reversal 100%) and normalised dilute thrombin time and ecarin clotting time in 88% to 98% of people.[181]
Andexanet alfa (recombinant coagulation factor Xa) is licensed to reverse the effect of apixaban and rivaroxaban. Andexanet alfa is licensed in the UK for use only in life-threatening or uncontrolled bleeding under specialist supervision.
Andexanet alfa quickly reverses the anticlotting effects of factor Xa inhibitors, according to an industry-supported study published in the New England Journal of Medicine.
Researchers enrolled 350 adults who presented with acute major bleeding (e.g., intracranial, gastrointestinal) within 18 hours of receiving apixaban or rivaroxaban. Andexanet alfa rapidly reduced anti-factor Xa activity. For example, among patients who'd been receiving apixaban or rivaroxaban, the initial andexanet alfa bolus reduced anti-factor Xa activity by 92%. In addition, at 12 hours after the infusion, 82% of patients were deemed to have good or excellent haemostatic efficacy.[182]
Some evidence has shown that rivaroxaban is safe and effective for outpatient management of low-risk PE.[183]
An international, multicentre single-arm clinical trial in 525 patients with acute PE who were taking rivaroxaban and were discharged within 2 days of PE diagnosis showed:[183]
Non-fatal VTE recurrence in 0.6% (one-sided upper 99.6% CI 2.1%)
Major bleeding in 1.2%.
This study did not use HESTIA or PESI/sPESI, instead using a different set of eligibility criteria for outpatient management.[183]
Haemodynamically stable PE (special patient groups)
Be aware of special cases such as pregnancy, abnormal kidney function, and active cancer as these groups will need a specific type/dose of anticoagulant.[76] If the patient has triple positive antiphospholipid syndrome, seek advice from a haematologist.
Pregnancy
Use a weight-adjusted dose of LMWH in women who are (or may be) pregnant.[67]
It does not cross the placenta, and routine monitoring is not generally required.
Evidence: LMWH in pregnancy
LMWH is safe and well tolerated in pregnant women.
Several studies show that heparin is safe to use in pregnancy.
A systematic review and a meta-analysis of the literature was carried out to provide an estimate of the risk of bleeding complications and VTE recurrence in patients with acute VTE during pregnancy treated with either LMWH or UFH. Eighteen studies (a total of 981 pregnant patients with acute VTE) were included. It concluded that LMWH and UFH appear to be safe and effective for the treatment of pregnancy-related VTE, but the optimal dosing regimens remain uncertain.[184]
A case series of 33 women showed that the initial dose of enoxaparin provided satisfactory peak anti-Xa activity. No woman developed thrombocytopenia, haemorrhagic complication, or further thromboembolic episode. Fifteen women had regional anaesthesia for delivery, with a reduced dose of enoxaparin, all without complication.[185]
In a retrospective observational study, enoxaparin was administered for treatment of an acute episode in 49 cases and for thromboprophylaxis in 574 cases. Serious maternal haemorrhage occurred in 11 cases during pregnancy (1.8%), one being reasonably related to enoxaparin, and in nine cases at delivery (1.4%), all unrelated to enoxaparin. Maternal thrombocytopenia was reported in 10 cases (1.6%), two being serious but unrelated to enoxaparin. Eight pregnancies ended in stillbirth (1.1%). Among the 693 live births, 17 major congenital abnormalities (2.5%) and 10 serious neonatal haemorrhages (1.4%) were reported. None of the fetal or neonatal adverse events were related to enoxaparin. Eight venous thromboembolic events (1.3%) were reported. The incidence of adverse events reported could be explained by the high risk profile of the study population. Overall, this retrospective study suggests enoxaparin is well tolerated during pregnancy.[186]
Avoid DOACs and warfarin during pregnancy as they may cross the placenta.[67]
Warfarin is associated with a well-defined embryopathy during the first trimester. Administration of warfarin in the third trimester can result in fetal and neonatal haemorrhage, as well as placental abruption. Warfarin may be associated with central nervous system anomalies throughout pregnancy.[67]
DOACs are not recommended because there is a lack of evidence of their safety in pregnancy as pregnant women were not included in trials.
However, warfarin, LMWH, and UFH are compatible with breastfeeding because they do not accumulate in breast milk and do not lead to anticoagulation in the infant.[187]
Active cancer
Check local protocols for these patients. The UK National Institute for Health and Care Excellence (NICE) suggests using a DOAC as first line.[76] Take into account the tumour site and interactions with other drugs, including those used to treat the cancer and the patient’s bleeding risk.[67][76] If these are unsuitable, other options recommended are:[76]
LMWH alone
OR
LMWH overlapped with warfarin. Ensure overlap with LMWH for at least 5 days or until the INR is ≥2 for at least two consecutive readings (whichever is the longer), followed by a VKA on its own.
Start anticoagulation in the acute phase and continue it for 3 to 6 months.[76]
Evidence: Anticoagulation for patients with active cancer
DOACs are at least as effective as LMWH for the initial treatment of VTE in people with active cancer, and in general do not seem to increase major or clinically relevant non-major bleeding.
Due to emerging data for the use of DOACs as initial treatment of VTE in people with active cancer, in 2023 NICE in the UK updated its systematic review of the evidence.[76] NICE found two randomised controlled trials (RCTs) specifically in people with cancer and VTE:
A 2018 UK multicentre RCT (SELECT-D) compared rivaroxaban with LMWH in 406 patients with active cancer with VTE (72% had symptomatic or incidental PE).[188]
It found rivaroxaban was associated with relatively low VTE recurrence (HR 0.43 [95% CI 0.19 to 0.99], high-quality evidence as assessed by GRADE) compared with LMWH, but higher clinically relevant non-major bleeding (HR 3.76 [95% CI 1.63 to 8.69], GRADE moderate) during the treatment period (up to 6 months).
There was no difference in other outcomes, including major bleeding and all-cause mortality, although the latter probably reflected most deaths being cancer related.
A 2018 multinational RCT (HOKUSAI-Cancer, 114 centres in 13 countries) compared edoxaban (preceded by 5 days of LMWH) with LMWH for the initial treatment of VTE in people with cancer (n=1046, 97% defined as active cancer).[189]
It found an increase in major bleeding (RR 2.01 [95% CI 1.12 to 3.61], GRADE low) and clinically relevant non-major bleeding (RR 1.46 [95% CI 1.03 to 2.07]) during the on-treatment phase (up to 12 months) in people offered edoxaban compared with LMWH.
There was no difference in VTE-recurrence up to 6 months or all-cause mortality up to 6 months (GRADE very low).
Three other relevant studies were subgroup analyses of the main DOAC studies.[76]
All were statistically underpowered and important patient information was not reported (e.g., primary cancer, number of people with metastatic cancer in each arm).
None of the studies showed any difference in outcomes with DOAC compared with LMWH.
Overall, there were insufficient data to be able to assess separately outcomes for people with pulmonary embolism or DVT.[76]
Since the final search date for the NICE guideline, two RCTs have been published comparing apixaban with LMWH in people with active cancer (ADAM VTE and the Caravaggio trial).[190][191]
ADAM VTE (n=300) found lower VTE recurrence (HR 0.099, 95% CI 0.013 to 0.780) and major bleeding rates (0% vs. 1.4%) with apixaban.[190]
A combined secondary safety outcome (major bleeding or clinically relevant non-major bleeding) was the same (6%) for both groups.
The Caravaggio trial was an open-label, multinational, non-inferiority study (n=1155).[191]
It found no difference in VTE recurrence (HR 0.63, 95% CI 0.37 to 1.07) or major bleeding (HR 0.82, 95% CI 0.40 to 1.69).
Severe abnormal kidney function or established kidney failure[76]
Seek advice from a haematologist for these patients. The UK National Institute for Health and Care Excellence (NICE) suggests the following based on estimated creatinine clearance (CrCl):[76]
For patients with CrCl 15-50 mL/minute, offer one of:
Apixaban (use caution if CrCl is 15-29 mL/minute)
Rivaroxaban (use caution if CrCl is 15-29 mL/minute)
LMWH or UFH which can be overlapped with warfarin or used as lead-in therapy before starting edoxaban. They can also be used as lead-in therapy before starting dabigatran if CrCl is ≥30 mL/minute.
For patients with CrCl <15 mL/minute offer one of:
LMWH alone
UFH alone
LMWH or UFH which can be overlapped with warfarin.
Practical tip
Warfarin is safe to use in patients with abnormal kidney function with no dose adjustments necessary. However, monitor the INR more carefully in these patients.
Recurrent PE
Seek advice from haematology for any patient who has a recurrent PE despite adequate anticoagulation treatment. Options include:[76]
Increasing the dose of anticoagulant
Changing to a different anticoagulant with a different mode of action.
Recurrent PE is unusual among patients receiving therapeutic-dose anticoagulation.[166]
Check adherence to anticoagulation treatment.[76]
Address other sources of hypercoagulability (e.g., underlying malignancy).[76]
Patients with recurrent PE despite treatment with adequate anticoagulation can be considered for a venous filter.[76]
Evidence: Risk of recurrence
The risk of recurrent VTE after discontinuation of anticoagulation depends on whether the PE was provoked or unprovoked.
The risk for recurrent VTE after discontinuation of anticoagulation is related to the features of the first VTE event.
A study that followed patients with a first episode of acute PE found that the recurrence rate after discontinuation of treatment was approximately 2.5% per year after PE associated with reversible risk factors compared with 4.5% per year after unprovoked PE.[192]
Similar observations were made in other prospective studies in patients with DVT.[193]
Recurrence rates may be up to 10% in the first year after withdrawal of anticoagulant treatment.
Urgent primary reperfusion is indicated for any haemodynamically unstable patient with:[67]
PE confirmed on computed tomographic pulmonary angiography (CTPA)
Suspected PE and unequivocal signs of right ventricular (RV) dysfunction on echocardiography (if CTPA is not immediately available and/or the patient is too unwell to undergo CTPA).
Thrombolysis is the first-line intervention for most patients.
Thrombolysis
Ideally, PE should be confirmed by CTPA prior to thrombolysis.[194] In practice, thrombolysis is typically started on clinical grounds if a high-risk PE is highly suspected and there is evidence of RV dysfunction on echocardiography.
Administer thrombolysis (unless contraindicated) to any haemodynamically unstable patient.[67][76]
Systemic thrombolysis is associated with lower all-cause mortality in these patients when compared with unfractionated heparin (UFH) alone.[67][138]
Consider thrombolysis in pregnant women after weighing up the risks and benefits.[67]
Give thrombolysis as soon as possible and certainly within 48 hours of symptom onset to ensure optimum benefit.[67]
Select an accelerated regimen administered over 2 hours.
These regimens are preferred to prolonged infusions over 12 to 24 hours. The European Society of Cardiology guideline lists alteplase, streptokinase, and urokinase as approved thrombolytics for acute PE.[67] Alteplase and higher-dose streptokinase are administered over 2 hours, whereas lower-dose streptokinase and urokinase are administered over 12 to 24 hours.
Give a vasopressor such as noradrenaline (norepinephrine) or adrenaline (epinephrine), or an inotrope such as dobutamine concurrently with, or while waiting for, thrombolysis if the patient remains haemodynamically unstable despite adequate intravenous fluids.[67]
Evidence: Thrombolysis outcomes
Most patients with high-risk PE respond to early thrombolysis.
Thrombolysis in the treatment of acute haemodynamically unstable PE has been shown to restore pulmonary perfusion more rapidly than anticoagulation with UFH alone.
A randomised controlled trial of 36 patients concluded that use of alteplase, compared with heparin, resulted in a greater and faster improvement of angiographic and haemodynamic variables. However, the high frequency of bleeding observed with alteplase in this trial suggests that patients should be carefully selected before thrombolytic therapy is given.[195]
Overall, >90% of patients appear to respond favourably to thrombolysis.
This was demonstrated in a prospective single-centre registry of 488 PE patients who underwent thrombolysis as judged by clinical and echocardiographic improvement within 36 hours.[196]
Among the minority of patients with high-risk PE who did not respond to thrombolysis, rescue surgical embolectomy led to lower mortality rates and fewer recurrent PEs when compared with repeat thrombolysis.
Thrombolysis has greatest benefit when given early after symptom onset.
An overview of 308 PE patients from five clinical trials who underwent thrombolysis for PE showed an inverse relation between duration of symptoms and improvement on post-treatment lung scan reperfusion scores. For each additional day of symptoms before thrombolysis, there was a decrease of 0.8% in lung tissue reperfusion. Similarly, on angiography, less clot lysis was observed immediately after thrombolysis in the group with the longest duration of symptoms compared with those with the shortest symptom duration.
However the authors concluded that thrombolysis may still have some benefit in patients who have had symptoms for 6 to 14 days.[197]
Evidence: Vasoactive drug selection
Choice of vasoactive drug is based on limited evidence.
Evidence suggests noradrenaline (norepinephrine) appears to improve right ventricular (RV) function through its inotropic effect, as well as improving coronary perfusion by raising systemic pressure.
However, noradrenaline increases pulmonary vascular resistance and no conclusive data are available regarding its potential use in PE.[198]
The use of dobutamine (an inotrope) may be considered for patients with PE, low cardiac index, and normal blood pressure based on the results of some studies.[67]
Dobutamine enhances contractility with an increase in stroke volume and cardiac output. However, raising the cardiac index above physiological values may aggravate the ventilation-perfusion mismatch by further redistributing flow from (partly) obstructed to unobstructed vessels.[67][199]
Adrenaline (epinephrine) combines the beneficial properties of noradrenaline (vasoconstriction with increased RV perfusion, positive inotropy) and dobutamine (positive inotropy), but without the vasodilatory effects associated with the latter.[67]
A small prospective, descriptive study of patients with RV failure and shock demonstrated that during adrenaline infusion, mean arterial pressure, cardiac index, and stroke volume index were increased, and RV ejection fraction improved.[200]
Another small study showed that adrenaline improved cardiac output in patients with shock without having a detrimental effect on pulmonary vascular resistance.[201]
Contraindications to thrombolysis
Seek haematology advice if a patient with high-risk PE who is haemodynamically unstable has any contraindications to thrombolysis.[67]
Absolute contraindications:
Haemorrhagic stroke or stroke of unknown origin at any time
Ischaemic stroke in the preceding 6 months
Central nervous system damage or neoplasms
Recent major trauma/surgery/head injury (in the preceding 3 weeks)
Gastrointestinal bleeding within the last month
Known bleeding risk.
Relative contraindications:
Transient ischaemic attack in the preceding 6 months
Oral anticoagulant therapy
Pregnancy or within 1 week postnatally
Traumatic resuscitation (in relation to this episode of PE)
Refractory hypertension (systolic blood pressure >180 mmHg)
Advanced liver disease
Infective endocarditis
Active peptic ulcer.
Practical tip
In practice, almost any contraindication to thrombolysis should be considered only relative in high-risk patients who present with haemodynamic instability.
This is because the mortality risk from high-risk PE is so high that it is likely to outweigh any bleeding risk from thrombolysis in this patient group.[67]
A decision on the risk-benefit balance should be made by a haematologist.
Evidence: Risks of thrombolysis
Thrombolysis carries a significant risk of major bleeding, including intracranial bleeding.
Analysis of pooled data from trials using various thrombolytic agents and regimens reported intracranial bleeding rates between 1.9% and 2.2%.[202][203]
Increasing age and the presence of comorbidities have been associated with a higher risk of bleeding complications.[204]
The PEITHO trial showed a 2% incidence of haemorrhagic stroke after thrombolytic treatment with tenecteplase (vs. 0.2% in the placebo arm) in patients with intermediate-high-risk PE. Major non-intracranial bleeding events were also increased in the tenecteplase group, compared with placebo (6.3% vs. 1.5%; P <0.001).[205] Note that tenecteplase is not licensed for use in PE.
These results underline the need to improve the safety of thrombolytic treatment in patients at increased risk of intracranial or other life-threatening bleeding.
A strategy using reduced-dose alteplase appeared to be safe in the setting of ‘moderate’ PE in a study that included 121 patients.[206] Another trial on 118 patients with haemodynamic instability or ‘massive pulmonary obstruction’ reported similar results.[207]
An alternative approach may consist of local, catheter-delivered, ultrasound-assisted thrombolysis using small doses of a thrombolytic agent.[67]
Don’t give thrombolysis routinely to intermediate-high risk patients (see Risk stratification below).[67][76]
In these patients, use of systemic thrombolysis is associated with a mortality benefit but it significantly increases the risk of bleeding, including intracranial haemorrhage.[138]
These patients should receive anticoagulation and be monitored closely over at least 48 to 72 hours. Rescue thrombolysis should be given if they develop signs of haemodynamic instability.[67]
Evidence: Thrombolysis in intermediate-risk patients
Evidence does not support the routine use of thrombolysis in patients with intermediate-risk PE.
UK and European guidelines do not recommend giving thrombolysis routinely in patients with intermediate-risk PE.[67][76] Evidence suggests that thrombolysis carries an unacceptably high bleeding risk in this group.[205]
The intermediate-risk group is defined by a Pulmonary Embolism Severity Index (PESI) score of III-IV or a simplified PESI (sPESI) score ≥1 (see Risk stratification below).
The international PEITHO (Pulmonary Embolism Thrombolysis) trial compared a single intravenous bolus of tenecteplase plus heparin with placebo plus heparin in 1006 patients with confirmed PE, RV dysfunction detected by echocardiography or CT, and a positive troponin I or T test.
In the thrombolysis group, haemodynamic decompensation/collapse or death within 7 days occurred less frequently than in the group receiving heparin alone.
However, in the thrombolysis group, compared with the placebo group, there was also a higher incidence of haemorrhagic stroke (2.0% vs. 0.2%) and major non-intracranial bleeding (6.3% vs. 1.5%).
Surgical embolectomy or catheter-directed therapy
The use of these will depend on local expertise and availability. According to the European Society of Cardiology guideline, they are indicated in the following circumstances:[67]
Patients who are unable to receive thrombolytic therapy because of bleeding risk
Insufficient time for effective systemic thrombolysis
Failed thrombolysis.
Evidence: Surgical pulmonary embolectomy and catheter-directed therapy outcomes
Evidence is scarce so management will depend on local options and expertise.
There is no comparative data to guide the primary management of patients with high-risk (massive) PE and a strong contraindication to systemic thrombolysis.
However, some small studies have looked at outcomes from surgical pulmonary embolectomy and catheter-directed therapy.
Mortality rates following pulmonary embolectomy range from 4% to 27%.[208] In a small cohort of patients who underwent surgical pulmonary embolectomy for acute high-risk (massive) pulmonary thromboembolism, the 10-year survival rate was 84%.[209]
A meta-analysis of non-randomised trials of catheter-directed therapies reported a clinical success rate of 87% with an associated risk of major and minor complications of 2% and 8%, respectively.[210]
The extent of early RV recovery after low-dose catheter-directed thrombolysis appears comparable to that after standard-dose systemic thrombolysis whereas anticoagulation with heparin alone has little effect on improvement of RV size and performance within the first 24 to 48 hours.
In a randomised controlled trial of 59 intermediate-risk patients, when compared with treatment by heparin alone, catheter-directed ultrasound-accelerated thrombolysis (administering 10 mg of alteplase per treated lung over 15 hours) significantly reduced the subannular RV/LV dimension ratio between baseline and 24-hour follow-up without an increase in bleeding complications.[211][212][213]
Evidence: Extracorporeal membrane oxygenation (ECMO)
Guidelines suggest certain situations in which ECMO may be considered, based on low-quality evidence.
The 2019 European Society of Cardiology (ESC) guideline for the diagnosis and management of acute PE states that in patients with high-risk PE and refractory circulatory collapse or cardiac arrest, temporary use of ECMO can be considered with surgical or catheter embolectomy (if appropriate expertise and resources are available).[67]
This was based on case series evidence (class of recommendation IIb, which indicates conflicting evidence and/or divergence of opinion about usefulness/efficacy of the treatment; the usefulness/efficacy is less well established by evidence/opinion thant class IIa). The guideline states that no randomised controlled trials have been reported, and further evidence (e.g., from cohort studies) is needed to support the place of ECMO in the treatment of acute high-risk PE.[67]
The most recent case series reported retrospectively on 180 patients with high-risk PE who were treated in various different centres (52 patients had veno-arterial ECMO and 128 were managed without ECMO).[214]
Overall the 30-day mortality was high at 48.3% (95% CI 41% to 56%). Patients who had ECMO as part of their treatment had a more severe presentation with a worse prognosis.[214]
The mortality rate was lower for ECMO plus surgical embolectomy (29.4%, 95% CI 51% to 89%) than for ECMO plus fibrinolysis (76.5%, 95% CI 57% to 97%) or for ECMO alone (77.7%, 95% CI 59% to 97%; P = 0.004).[214]
The trialists concluded that the results indicate promise for ECMO when used in combination with surgical embolectomy in patients with high-risk PE, but no justifiable role for ECMO when used in patients with failed thrombolysis or as a stand-alone treatment.[214]
In this case series, 38.5% (95% CI 25% to 52%) of patients treated with ECMO (with or without other treatments) had a major bleeding event in hospital.[214]
The 2019 ESC guideline states that the increased risk of bleeding related to need for vascular access is an important consideration, particularly when patients are also undergoing thrombolysis.[67]
Venous filters
Consider a venous filter for any patient with confirmed PE who is deemed unsuitable for anticoagulation after discussion with a haematologist.[76] In practice, venous filters are rarely used outside the US and their use remains controversial.[194]
Many of these patients (even those with apparent absolute contraindications to anticoagulation) may still be able to have a different or altered dose of anticoagulation with an acceptable risk-benefit balance. This is usually preferred to a venous filter.
Evidence: Venous filters
The evidence shows varied outcomes from use of venous filters in PE.
The use of venous filters is controversial and there is limited evidence to support recommendations about their use in clinical practice. For use in acute PE the evidence shows varied outcomes.
There have only been two randomised controlled trials performed in Europe.
The first, PREPIC (Prévention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group), randomised 400 patients who suffered acute venous thromboembolism (VTE) to anticoagulation alone or anticoagulation and a permanent inferior vena cava (IVC) filter. Follow-up at 8 years showed that while IVC filters reduced the risk of recurrent PE, they did not prevent death, and more deep vein thrombosis (DVT) occurred in patients who received these devices.
The second study, PREPIC 2, randomised 399 patients with PE associated with DVT to anticoagulation alone or anticoagulation plus a retrievable IVC filter. All the patients had at least one ‘high-risk’ feature (age >75 years, active cancer, chronic cardiac or respiratory insufficiency, ischaemic stroke with leg paralysis within 6 months, either iliocaval or bilateral DVT, or a sign of RV dysfunction or myocardial injury). Again, use of venous filters did not show any mortality benefit nor did they result in fewer symptomatic PEs during the first 3 months of follow-up.
On the other hand, observational studies from the US, analysing almost 298,000 filter implantations, suggest that insertion of a venous filter might reduce PE-related mortality rates in the acute phase, with benefit possibly coming at the cost of an increased risk of recurrence of VTE.[215][216]
Risk stratify haemodynamically stable patients using the Pulmonary Embolism Severity Index (PESI) score or the simplified Pulmonary Embolism Severity Index (sPESI) score to determine which patients may be safely discharged and which need admission with closer monitoring.[67][76][77][106] [ Pulmonary Embolism Severity Index (PESI) Opens in new window ]
Do not use PESI/sPESI scoring in:
Patients with hypotension or shock. All such patients are high-risk and must be managed accordingly with urgent primary reperfusion and anticoagulation
Pregnant women
Patients with active cancer; use the HESTIA score instead.[106]
Intermediate-risk PE
A patient is considered intermediate-risk if they have a PESI score of III-IV or an sPESI score of ≥1.[67][106]
Risk stratify these patients further by measuring both their right ventricular (RV) function (on computed tomographic pulmonary angiography [CTPA] or echocardiography) and a cardiac biomarker.[67]
Patients are classed as intermediate-high risk if:[67]
There are signs of RV dysfunction
AND
The cardiac biomarker is elevated.
Start anticoagulation and monitor intermediate-high risk patients very closely as they are at risk of deterioration. If they become haemodynamically unstable, they will need reperfusion therapy (usually rescue thrombolysis).[67]
Patients are classified as intermediate-low risk (and should be admitted but do not require close monitoring) if there are:[67]
No signs of RV dysfunction AND the cardiac biomarker is normal
OR
Either signs of RV dysfunction OR the cardiac biomarker is elevated.
Consider early discharge once a patient admitted with an intermediate-risk PE improves sufficiently to meet the criteria for low-risk PE (PESI I-II or sPESI 0).[106]
Low-risk PE
A patient is considered low-risk if they have a PESI score of I-II or an sPESI score of 0.[67][106]
Consider outpatient management in patients who are classified as low risk.[76]
PE does not need to be confirmed on CTPA prior to discharge but it should be performed within 24 hours (if available).[106]
Assessment of RV size/function on CTPA or echocardiography is not essential to confirm a patient as low-risk and suitable for outpatient management.[106]
The 2018 British Thoracic Society (BTS) guideline states that echocardiography does not increase the predictive power of validated risk scores such as PESI/sPESI or HESTIA.
It recommends considering measurement of cardiac biomarkers (high-sensitivity troponin, B-type natriuretic peptide [BNP], or N-terminal pro-B-type natriuretic peptide [NT-proBNP]) only in patients who had evidence of RV dysfunction on CTPA/echo if performed during diagnostic work-up.
If these are normal the patient can be considered for discharge. If they are elevated they should be admitted for observation.[106]
Do not discharge any patient with a low-risk sPESI score who meets one or more of these exclusion criteria:[106]
Haemodynamic instability
The exclusion criteria define this as heart rate >110 bpm, systolic blood pressure (SBP) <100 mmHg, requirement for inotropes and critical care, or requirement for thrombolysis or embolectomy. However, bear in mind that the 2019 European Society of Cardiology guideline uses a different definition of haemodynamic instability. See Diagnosis recommendations.
Oxygen saturations <90% on air
Active bleeding or risk of major bleeding (e.g., recent gastrointestinal bleed or surgery, previous intracranial bleeding, uncontrolled hypertension)
On full-dose anticoagulation at the time of the PE
Severe pain (e.g., requiring opioid analgesics)
Other medical comorbidities requiring hospital admission
Chronic kidney disease stages 4 or 5 (estimated glomerular filtration rate <30 mL/minute) or severe liver disease
Heparin-induced thrombocytopenia within the last year and where there is no alternative to repeating heparin treatment
Social reasons, which may include inability to return home, inadequate care at home, lack of telephone communication, concerns over compliance, etc.[106]
Practical tip
HESTIA is an alternative validated risk score that is used instead of PESI/sPESI to select patients with active cancer who are suitable for outpatient management of PE.[106]
If any one or more of the HESTIA exclusion criteria is present, the HESTIA score is considered positive and patients are deemed unsuitable for discharge.[106]
The HESTIA exclusion criteria are very similar to the BTS exclusion criteria for patients with a low-risk sPESI score:
Haemodynamic instability
The HESTIA criteria define this as SBP <100 mmHg with heart rate >100 bpm or condition requiring admission to an intensive care unit. However, bear in mind that the 2019 European Society of Cardiology guideline uses a different definition of haemodynamic instability. See Diagnosis recommendations.
Thrombolysis or embolectomy indicated
Active bleeding or high risk of bleeding
Gastrointestinal bleeding in the preceding 14 days
Recent stroke (<4 weeks ago)
Recent operation (<2 weeks ago)
Bleeding disorder
Thrombocytopenia (platelet count <75 x 109/L)
Uncontrolled hypertension (SBP >180 mmHg or diastolic BP >110 mmHg)
Oxygen required for >24 hours to maintain oxygen saturations >90%
PE diagnosed during anticoagulant treatment
Severe pain requiring intravenous pain medication for >24 hours
Medical or social reason for treatment in the hospital for >24 hours
Creatinine clearance <30 mL/minute
Severe liver impairment (at discretion of clinician)
Pregnancy
Documented history of heparin-induced thrombocytopenia.
Evidence: sPESI vs. HESTIA
sPESI and HESTIA risk scores perform similarly in identifying patients at risk of poor outcomes.
One study compared the Hestia criteria with sPESI for identifying patients at risk of 30-day mortality. Of 468 patients, 53% were identified as suitable for outpatient management using the Hestia exclusion criteria, with 59% deemed low risk according to sPESI.[217]
Although both tools selected slightly different patients as low risk, they had similar overall outcomes, with 30-day adverse events (major bleeding, recurrent venous thromboembolism, or death) of 2.4% in the Hestia group and 2.2% in the sPESI low-risk group. sPESI performed slightly better than Hestia in testing.
Discharging patients
Request a consultant review prior to discharge of any patient with confirmed or suspected PE.[106]
If no consultant is available, then patients may be reviewed by a senior trainee (ST3 or above; ST4 in the case of emergency medicine), or by a staff grade or similar substantive career grade doctor, advanced nurse practitioner, or clinical nurse specialist designated to undertake this role within the department with consultant advice available.
Provide verbal and written information on the signs and symptoms of recurrence, major bleeding, and additional complications.[76][106]
There should also be an appropriate point of contact in the event of complications or concerns, both in and out of hours.[76]
Organise a formal review (telephone/face-to-face) at least once during the first week after discharge to ensure therapeutic compliance and check for any complications. There should be local protocols and pathways in place for follow-up of all patients with PE, whether treated as an inpatient or outpatient.[106]
This should include assessment of ongoing symptoms (with further directed investigation as appropriate) and consideration of optimal duration/type of anticoagulation.
Duration of ongoing anticoagulation
Continue anticoagulation for at least 3 months for all patients with PE. Review all patients at 3 months as a minimum.[67][218]
Discuss with a senior colleague whether to continue anticoagulation beyond 3 months. This decision should weigh up the individual patient’s risk of recurrence of PE versus bleeding risk. Discuss the risks and benefits of long-term anticoagulation with the patient, and take their preferences into account.[76]
In general, anticoagulation can usually stopped after 3 months (or 3 to 6 months for people with active cancer) if the PE was provoked, as long as the transient risk factor is no longer present and the clinical course has been uncomplicated. Anticoagulation is usually continued for longer if the PE was unprovoked.[76]
A provoked PE is one associated with a transient risk factor that was present in the 3 months prior to the PE.[76]
An unprovoked PE is a PE in a patient who had no pre-existing, transient provoking risk factor in the prior 3 months.[76]
Provoking risk factors include: surgery; trauma; significant immobility (bedbound, unable to walk unaided, or likely to spend a substantial proportion of the day in bed or in a chair); pregnancy or puerperium; use of oral contraceptive/hormone replacement therapy).[76]
Evidence: Duration of anticoagulation
There is good evidence for longer duration of anticoagulation in unprovoked compared with provoked PE.
Clinical trials have evaluated various durations of anticoagulant treatment for venous thromboembolism (VTE). The main findings of these studies were:[193][219][220][221]
Patients with PE should receive at least 3 months of anticoagulant treatment.
The risk of recurrence if anticoagulants are stopped after 6 or 12 months can be expected to be similar to that after 3 months.
Indefinite treatment reduces the risk for recurrent VTE by about 90%, but this benefit is partially offset by a 1% or higher annual risk of major bleeding.
Evidence shows 3 months is the optimum duration of anticoagulation treatment in patients with a provoked PE.
Four randomised controlled trials (RCTs) compared 3 months of anticoagulation with 6 to 12 months of therapy.[192][222][223] Meta-analysis of their findings found a similar risk of recurrence with 3 months compared to 6 to 12 months of therapy during 1 to 3 years of follow-up.[224] Analysis of individual patient data from these four trials, and another study that compared 3 months with 27 months of anticoagulation, also found no convincing increase in the risk of recurrence after treatment was stopped after 3 months.[225][226]
Five RCTs compared 4 to 6 weeks of anticoagulation with 3 to 6 months of anticoagulation.[221][222][227][228][229] Meta-analysis of their findings found that the shorter course of therapy was associated with about a twofold increase in recurrence during 9 to 24 months of follow-up. Analysis of individual patient data from four of these trials demonstrated that the risk of recurrence after stopping anticoagulant therapy was higher in patients who were treated for 4 to 6 weeks than in those treated for 3 months or more.[224] Furthermore, the excess recurrences with 4 to 6 weeks of therapy were confined in the first 6 months after stopping therapy and, in those with a deep vein thrombosis, the extra recurrences were in the same leg as the initial event.[226]
There is also growing evidence that longer anticoagulation in unprovoked PE is beneficial in order to reduce recurrence.
Extended anticoagulation with warfarin has been shown to reduce recurrent VTE by approximately 90% based on meta-analysis of four studies, with about half of the recurrent episodes occurring in patients who had prematurely stopped therapy.[193][224][225][230][231]
Direct and indirect comparisons have found similar reductions in recurrent VTE with extended anticoagulation using dabigatran, rivaroxaban, or apixaban.[178][232][233][234] Results from a systematic review and meta-analysis of 16 studies showed that longer anticoagulation with a vitamin K antagonist or a direct-acting oral anticoagulant (DOAC) reduced recurrent VTE but use of a DOAC was associated with a lower bleeding risk and mortality overall.[235]
Extended treatment with low molecular weight heparin is also very effective, and is more effective than warfarin in cancer patients.[224][236][237] Results from a systematic review and meta-analysis of 16 studies showed that longer anticoagulation with a vitamin K antagonist or a DOAC reduced recurrent VTE, but use of a DOAC was associated with a lower bleeding risk and mortality overall.[238]
Clinicians should balance the risk of bleeding against the risk of recurrence when making a decision about extended secondary prevention.[218]
Annually reassess the risks/benefits of continuing anticoagulation, as well as the patient's general health and treatment preferences, in all patients receiving extended treatment beyond 3 months.[67][76][166]
Evidence: Assessment of bleeding risk
Many studies examining major bleeding rates have assessed these over 3 months or longer, whereas bleeding in the first 7 to 14 days may be more relevant for risks related to outpatient PE management.
In the RIETE registry of 24,395 patients with VTE on anticoagulation, 2.24% had a major bleed and 0.55% had a fatal bleed during the first 3 months of anticoagulation. The RIETE investigators have derived and validated a score in patients with documented VTE to predict the risk of major bleeding within 3 months of anticoagulant therapy.[239] On multivariate analysis, age >75 years, recent bleeding, cancer, creatinine levels >1.2 mg/dL, anaemia, or PE itself were independently associated with an increased risk of major bleeding.
Another study compared the predictive value of the HEMORR2HAGES, HASBLED, and ATRIA scores to the Kuijer and RIETE scores for the occurrence of major bleeding complications over a 30-day period in 448 consecutive patients with PE treated with warfarin. Most bleeding events (16/20) occurred in the first 7 days after treatment initiation, with four bleeding complications between days 8 and 30. The predictive power of all five scores for bleeding was poor (c-statistics 0.57–0.64), both for the three-level and two-level score outcomes. No individual score was found to be superior. The HASBLED score had a good c-statistic for bleeding occurring after the first week of treatment (0.75, 95% CI 0.47 to 1.0). There are no studies deriving or validating an early bleeding risk score specifically for outpatient PE management.[240]
In general, offer continued treatment with the anticoagulant used in the acute phase if it is well tolerated.[76]
If the patient has been started on a DOAC other than apixaban and this is not well tolerated, or the clinical situation or patient preference has changed, consider switching to apixaban if the patient does not have abnormal kidney function, active cancer, established triple positive antiphospholipid syndrome, or extreme body weight (<50 kg or >120 kg).[76]
In patients taking apixaban or rivaroxaban who need ongoing anticoagulation for >6 months, it is becoming common practice to consider a dose reduction.[241]
In pregnant patients, continue LMWH for the remainder of the pregnancy and for at least 6 weeks postnatally and until at least 3 months of treatment has been given in total.[242]
Consider aspirin if the patient refuses or is unable to tolerate any form of oral anticoagulant.[76]
Evidence: DOACs versus heparin/warfarin
DOACs have emerged as an equally effective and safer option than heparin/warfarin.
There is now strong evidence that DOACs are non-inferior and have a favourable safety profile for management of PE when compared to either low molecular weight heparin/warfarin or fondaparinux.[67][106]
Results of the major trials using DOACs in the treatment of VTE indicate that these agents are non-inferior (in terms of efficacy) and possibly safer (particularly in terms of major bleeding) than the standard heparin/warfarin regimen.[67][173][174][175][176][177][178][179] [
 ]
[Evidence B]
]
[Evidence B]A Cochrane review concluded there was:
No difference between dabigatran and the standard regimen in preventing recurrent PE (odds ratio [OR] 1.02, 95% CI 0.50 to 2.04), recurrent VTE (OR 0.93, 95% CI 0.52 to 1.66), and deep vein thrombosis (OR 0.79, 95% CI 0.29 to 2.13), or in causing major bleeding (OR 0.50, 95% CI 0.15 to 1.68).
No difference between the factor Xa inhibitors (apixaban, rivaroxaban, edoxaban), compared with the standard regimen, in preventing recurrent VTE (OR 0.85, 95% CI 0.15 to 1.68), DVT (OR 0.72, 95% CI 0.39 to 1.32), and all-cause mortality (OR 1.16, 95% CI 0.59 to 1.62), or in causing major bleeding (OR 0.97, 95% CI 0.59 to 1.62).
High time in therapeutic range values were achieved under warfarin treatment in all trials. On the other hand, the study populations included relatively young patients, very few of whom had cancer.
Evidence: Secondary prevention with warfarin
Warfarin for extended secondary prevention reduces mortality but carries a risk of bleeding.
A review of 22,000 patients demonstrated that standard-intensity warfarin reduced the all-cause mortality and reduced the risk of recurrent VTE compared with placebo or observation only when used for extended secondary prevention.
However, warfarin also increased the risk of bleeding, with one major bleed for every 87 people treated.[218]
Evidence: Aspirin for extended secondary prevention
Aspirin is less effective than oral anticoagulants for extended secondary prevention. However, it is more effective than placebo and therefore it may be considered for people who require extended treatment but who refuse or cannot tolerate oral anticoagulants.
In a 2023 guideline update the UK National Institute for Health and Care Excellence (NICE) assessed the evidence for extended use of aspirin in the secondary prevention of VTE.[76]
NICE found three RCTs:
Two studies published in 2012, the ASPIRE trial (n=822) and the WARFASA trial (n=403), compared aspirin with placebo for 24 to 48 months in people with unprovoked venous thromboembolism (ASPIRE: 28% PE alone, 14% DVT and PE; WARFASA: 40.5% PE in aspirin arm and 34.1% in placebo arm).[243][244]
The EINSTEIN-CHOICE 2017 trial (n=3365) compared aspirin with two different doses of rivaroxaban for up to 24 months; 34% of participants had PE alone and 15% DVT and PE. Overall, 41.3% had unprovoked VTE.[245]
NICE also conducted a network meta-analysis, which meant it was possible, via indirect comparisons, to compare aspirin with vitamin K antagonists and other DOACs.
Aspirin was not as effective as vitamin K antagonists or DOACs for the prevention of recurrent VTE.
However, aspirin was more effective than placebo at preventing recurrence (HR 0.68 [95% CI 0.51 to 0.90], moderate-quality evidence as assessed by GRADE), and it did not increase the risk of major bleeding compared with placebo (ASPIRE [up to 48 months]: RR 1.33 [95% CI 0.47 to 3.81], GRADE low; WARFASA [up to 24 months]: RR 0.96 [95% CI 0.06 to 15.26], GRADE very low).
The NICE guideline committee therefore recommends aspirin as an option for people requiring extended treatment for VTE who decline to take an oral anticoagulant, following an informed discussion of benefits and harms, especially regarding the individual risk of VTE recurrence.
The 2019 European Society of Cardiology guideline also recommends aspirin for extended secondary prevention for patients who refuse to take or are unable to tolerate any form of oral anticoagulants.[67]
Use of this content is subject to our disclaimer