Recommendations
Urgent
Assess for signs of haemodynamic instability (ABCDE approach) as this can indicate a high-risk massive/central PE with high risk of a poor outcome.
Seek senior support and escalate to critical care if you suspect a high-risk PE.[67][74]
Haemodynamic instability in PE is defined as at least one of the following at presentation:[67]
Cardiac arrest; need for cardiopulmonary resuscitation
Obstructive shock; systolic blood pressure (SBP) <90 mmHg or vasoactive drugs required to achieve a BP ≥90 mmHg despite adequate filling status AND end-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate)
Persistent hypotension; SBP <90 mmHg or SBP drop ≥40 mmHg, lasting longer than 15 minutes and not caused by new-onset arrhythmia, hypovolaemia, or sepsis.
If the patient is in peri-/cardiac arrest and PE is suspected it is common practice to give thrombolysis without waiting for results of investigations, based on discussion between senior clinicians.
Ensure early respiratory support. Give oxygen if saturations are <90%.[67]
Titrate oxygen to target saturations of 94% to 98% (or 88% to 92% for patients at risk of hypercapnic failure).[67]
Get an urgent senior review if SBP is <90 mmHg and jugular venous pressure (JVP) is elevated to determine whether intravenous fluids need to be given. Give intravenous fluids if SBP is <90 mmHg and the JVP is not elevated.[67]
In a patient with haemodynamic instability, urgently differentiate high-risk PE from other acute life-threatening causes using bedside echocardiography (emergency computed tomographic pulmonary angiography [CTPA] may be used if the patient is stable enough to undergo this).[67]
If signs of right ventricular (RV) dysfunction are seen on echocardiography or PE is confirmed on CTPA, arrange urgent primary reperfusion, usually with thrombolysis.
Key Recommendations
Using the Pulmonary Embolism Rule-Out Criteria (PERC) rule[Figure caption and citation for the preceding image starts]: Summary: pulmonary embolism diagnostic pathwayCreated by BMJ Knowledge Centre [Citation ends].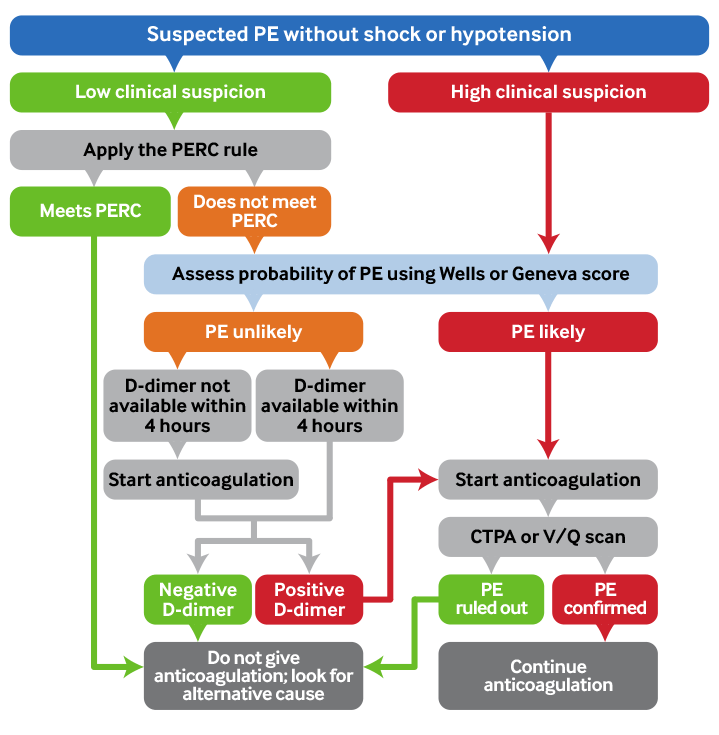
Presentation
Suspect pulmonary embolism (PE) in a patient with acute onset of dyspnoea, pleuritic chest pain, or features of deep vein thrombosis (DVT).
At least one of these features is present in 97% of people with PE.[75]
Symptoms typically come on suddenly rather than gradually.
Dyspnoea is the most common presenting feature and may be acute and severe in a central PE but is often mild and transient in a small, peripheral PE.
Around 95% of patients with PE are haemodynamically stable on presentation.
The 5% of patients who have a high-risk (haemodynamically unstable) PE will have features of hypotension, shock, or tachycardia and can be hypoxaemic at rest.
A patient with a PE may also present with cough or, more rarely, with fever or haemoptysis.[67]
Strong risk factors include pregnancy/6 weeks postpartum; active cancer; recent surgery or immobilisation; and a history of DVT.[67]
Assessing the clinical probability of PE
If you have a low clinical suspicion of PE, use the Pulmonary Embolism Rule-Out Criteria (the PERC rule). [76]
If you have a high clinical suspicion of PE or the patient does not meet the PERC rule, use the Wells (or Geneva) score to categorise the patient as ‘PE likely’ (Wells score >4) or ‘PE unlikely’ (Wells score ≤4).[67][76][77] [ Pulmonary Embolism Wells Score Opens in new window ] [ Revised Geneva Score for Estimation of the Clinical Probability of Pulmonary Embolism in Adults Opens in new window ]
Rule out PE in haemodynamically stable patients who present with a suspected PE using a combination of clinical probability assessment, the PERC rule, the Wells (or Geneva) score, and D-dimer testing (if indicated).[67]
If a patient has a ‘PE likely’ Wells (or Geneva) score, start anticoagulation then arrange investigation with definitive imaging (usually computed tomographic pulmonary angiography [CTPA] if available).
If a patient has a ‘PE unlikely’ Wells (or Geneva) score, order a D-dimer. Offer anticoagulation if the D-dimer result cannot be obtained within 4 hours.[76]
If D-dimer level is elevated, start anticoagulation and arrange CTPA to confirm or exclude a PE. A normal D-dimer level safely excludes a PE in a patient who had a ‘PE unlikely’ Wells (or Geneva) pre-test score.[76]
Diagnostic confirmation
Confirm diagnosis in most patients using CTPA, which will show direct visualisation of a thrombus in a pulmonary artery and appears as a partial or complete intraluminal filling defect.[67][74][76]
Avoid CTPA radiation in patients with a suspected concurrent DVT by using lower limb compression venous ultrasound.[74][76]
A ventilation-perfusion (V/Q) scan may be preferred to CTPA to minimise radiation exposure in certain patient groups. This is a radiation- and contrast medium-sparing procedure and should be used in patients with contraindications or relative contraindications to CTPA (e.g., contrast allergy, moderate to severe abnormal kidney function or established kidney failure, young patients).[67][76]
Suspected PE in pregnancy
Seek advice from a senior clinician if the patient with suspected PE is pregnant.
PE is the leading cause of maternal mortality in developed countries. There is a fourfold increased risk of venous thromboembolism (VTE) throughout pregnancy compared with non-pregnant people. This risk rises sharply in the postnatal period and is up to 60 times higher in the 3 months after delivery, compared with the risk in non-pregnant women.[67][78]
Symptoms and signs of PE may be less specific in pregnant women compared with non-pregnant people.[74] Be cautious in interpreting breathlessness as it is a common symptom in pregnancy.[67]
Use the YEARS criteria in combination with a D-dimer level to determine whether further definitive imaging is necessary.[67][79][80]
Order a chest x-ray if you suspect PE. If this is abnormal, then CTPA is the first-line option for definitive diagnosis of PE. If this is normal, then CTPA or V/Q scan should be considered, depending on local availability.[67]
Symptoms of PE typically have an acute rather than a gradual onset.[67]
Patients typically present with at least one of dyspnoea, pleuritic chest pain, or features of deep vein thrombosis (DVT).[67][76]
Dyspnoea
The most common presenting feature - it is present in 50% of patients who are subsequently found to have a PE.[67]
Dyspnoea may be acute and severe in a central PE but is often mild and may be transient in a small peripheral PE.[67]
Tachypnoea (respiratory rate ≥20 breaths per minute) is a common presenting sign in patients with acute PE (21% to 39%).[4]
Practical tip
In a patient with pre-existing heart failure or lung disease, worsening dyspnoea may be the only presenting symptom of a PE.[67]
Pleuritic chest pain
Seen at presentation in 39% of patients subsequently diagnosed with a PE.[67]
The pain is normally localised to one side of the chest and is unlikely to be central. This is usually caused by pleural irritation due to distal emboli causing pulmonary infarction.[81]
Central PE may also present with angina-like chest pain but this is uncommon. This may reflect right ventricular (RV) ischaemia and requires differential diagnosis with acute coronary syndrome or aortic dissection.[67]
Practical tip
Recurrent or transient episodes of pleuritic chest pain lasting minutes are very unlikely to be caused by multiple PEs.
Features of suspected DVT
Cough and hypoxaemia are also common.
Cough
Seen at presentation in 23% of patients subsequently diagnosed with a PE.[67]
Hypoxaemia
Hypoxaemia at rest may be suggestive of a massive PE whereas hypoxaemia solely on exertion is more often associated with a smaller PE.
Practical tip
A normal oxygen saturation does not exclude PE.
Up to 40% of patients have normal arterial oxygen saturation and 20% have a normal alveolar-arterial oxygen gradient.[67] [ A-a Gradient Opens in new window ]
Patients with a massive/central PE, or those with a smaller PE but who have significant pre-existing cardiorespiratory impairment, typically present with:
Syncope or pre-syncope
Seen at presentation in 6% of patients with PE.[67]
Hypotension
Defined as systolic blood pressure <90 mmHg.[67]
Other features of shock[67]
Look for signs of altered cognition. Agitation and distress characterise early and milder stages, whereas unresponsiveness indicates more severe and advanced shock.
Check for cool extremities, mottled or ashen skin, slow capillary refill, clamminess, and oliguria.
Tachycardia
Heart rate >100 bpm.
Tachycardia is an important sign of a possible high-risk PE even in the absence of other signs of shock.
Rarer features of PE:
Fever
Haemoptysis
Present in 8% of patients with PE.[67]
It is commonly estimated that around half of patients presenting with apparent haemoptysis do not have true haemoptysis. Instead this could be epistaxis or blood from the gastrointestinal tract, upper airways, or gingiva. It is vital to take a clear and thorough history and examination to distinguish this.
Practical tip
A PE is unlikely to be the cause in a patient who has an episode of syncope or pre-syncope but subsequently has normal observations, is haemodynamically stable and has no abnormality suggestive of a PE on their ECG.
Clinical feature | % of patients with subsequently confirmed PE who had the clinical feature at presentation[67] |
|---|---|
Dyspnoea | 50% |
Pleuritic chest pain | 39% |
Signs of DVT (unilateral leg swelling) | 24% |
Cough | 23% |
Substernal chest pain | 15% |
Fever | 10% |
Haemoptysis | 8% |
Syncope | 6% |
Unilateral leg pain | 6% |
Your history should check for the following risk factors to help assess the likelihood of PE and to determine whether any subsequently confirmed PE is provoked or unprovoked:[34][60][66][67][76]
No provoking cause can be found in 30% of patients.[67]
Strong risk factors | Weak risk factors | |
|---|---|---|
| Past medical history | Active cancer
Recent surgery or hospitalisation (last 3 months)
Previous or current deep vein thrombosis (DVT)
Pregnancy and 6 weeks postpartum[34][67][78]
| Other significant medical comorbidities
Obesity
Increasing age Varicose veins Known thrombophilias
Other
|
| Social history | History of immobilisation
| Long-distance travel Smoking |
| Drug history | Combined oral contraceptive pill
Hormone replacement therapy[76]
| |
| Family history | First-degree relative with a history of confirmed PE or DVT[76] |
Evidence: Cancer as a risk factor
Cancer significantly increases the risk of a PE.
Evidence suggests that the overall risk of venous thromboembolism (VTE) in cancer patients is four times higher than in the general population.[88]
Although the largest absolute number of VTE episodes occur in patients with lung, colon, and prostate cancer, a population-based cohort study demonstrated that the relative risk for VTE was highest in multiple myeloma, brain, and pancreatic cancer (46-, 20- and 16-fold increased risk vs. healthy controls, respectively).[83]
In the metastatic stage, there is evidence that stomach, bladder, uterine, renal, and lung cancer are also associated with a high incidence of VTE.[89]
Physical examination is often non-specific unless a PE is massive/central. However, on examination you should check for:
Signs of a concurrent deep vein thrombosis (DVT). Examine the lower limbs for tenderness, changes to skin colour and temperature, and venous distension.[82]
Hypoxaemia. This may be present only on exertion in smaller PEs but the patient may appear tachypnoeic.[76]
Crepitations on auscultation of the lungs.
Fever. Temperature >37.8°C.[3]
Practical tip
It is possible for PE and pneumonia to co-exist.
Fever may be present in both but tends to be higher (>39°C) in pneumonia.[90]
It is important to note that chest pain on palpation of the chest wall occurs in up to 20% of people with PE.
Therefore do not use this finding to distinguish musculoskeletal chest pain from PE.[91]
Look for features of a massive/central PE and/or severely reduced haemodynamic reserve including:
Haemodynamic instability (present in less than 5% of patients with acute PE[92])[67]
Cardiac arrest; need for cardiopulmonary resuscitation
Obstructive shock; systolic blood pressure <90 mmHg or vasoactive drugs required to achieve a BP ≥90 mmHg despite adequate filling status AND end-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate[67][76]
Persistent hypotension; systolic blood pressure <90 mmHg or systolic blood pressure drop ≥40 mmHg, lasting longer than 15 minutes and not caused by new-onset arrhythmia, hypovolaemia, or sepsis
Tachycardia (heart rate >100 bpm). This is an important sign of a possible high-risk PE even in the absence of other signs of shock
Signs of acute right ventricular (RV) dysfunction.[77] The patient may have an elevated jugular venous pressure, parasternal heave, or accentuated pulmonary component of S2, although this is uncommon.[93]
The symptoms and signs of PE are non-specific and no individual risk factor, patient symptom, or clinical sign can definitively diagnose or exclude PE.[67]
Assessment of clinical probability is therefore a key step in the diagnostic approach.
Assess the clinical probability of PE in haemodynamically stable patients using a combination of the Pulmonary Embolism Rule-Out Criteria (the PERC rule), the Wells (or Geneva) score, plus D-dimer testing if indicated.[67][76] [ Pulmonary Embolism Wells Score Opens in new window ] [ Revised Geneva Score for Estimation of the Clinical Probability of Pulmonary Embolism in Adults Opens in new window ]
This probability-based approach minimises the unnecessary use of potentially harmful computed tomographic pulmonary angiography (CTPA) in patients who have a low probability of a PE.[94]
If you have a low clinical suspicion of PE, use the PERC rule.[76]
PE can effectively be ruled out in any patient who meets the PERC rule.[74]
If you have a high clinical suspicion of PE or the patient does not meet the PERC rule, use the Wells (or Geneva) score to categorise the patient as 'PE likely' or 'PE unlikely' . An alternative approach is to use D-dimer adjusted to age for patients aged over 50 years. Follow local protocols.[67][76]
Start anticoagulation and arrange CTPA for any patient in the 'PE likely' group.
Pulmonary Embolism Rule-Out Criteria (the PERC rule)
If you have a low clinical suspicion of PE, apply the PERC rule.[76]
No further investigation is indicated if a patient meets the PERC rule (a PE can effectively be ruled out). The risk of PE is considered to be lower than the risk of testing.[74]
Request D-dimer testing for any patient in whom the PERC rule fails to exclude PE (i.e., one or more criteria not fulfilled).[95]
The PERC rule is:[96]
Age <50 years
Heart rate <100 bpm
SaO 2 on room air ≥95%
No unilateral leg swelling
No haemoptysis
No recent surgery or trauma (≤4 weeks ago requiring treatment with general anaesthesia)
No prior PE or DVT
No hormone use (oral contraceptives, hormone replacement, or oestrogenic hormones used in male or female patients).
Be aware that the PERC rule has not been validated in people with COVID-19.[76]
Evidence: The PERC rule
Use of the PERC rule can help prevent over-investigation when clinical suspicion of PE is low.
The UK National Institute for Health and Care Excellence (NICE) recommends considering using the PERC rule to determine whether any further investigations are required where there is a low clinical suspicion of PE (based on history, examination, and initial investigations such as ECG and chest x-ray) and other diagnoses are feasible. This recommendation is based on a 2020 NICE evidence review that assessed the diagnostic accuracy of the PERC rule.[97]
The evidence review included 7 studies (1 large cluster randomised controlled trial [RCT; people with very low pre-test probability of PE, n=1916] and 6 diagnostic accuracy studies [n=5690]).
All of the diagnostic accuracy studies were prospective cohorts; however, the performance of the PERC rule was often assessed retrospectively in these studies.
The evidence was of low to very low quality as assessed by GRADE due to lack of blinding of assessors (in some of the diagnostic accuracy studies) and of clinicians (in the RCT). There was also high heterogeneity in the diagnostic accuracy studies and very serious imprecision (due to very low event rates in both groups) in the RCT.
Meta-analysis of the diagnostic test accuracy studies found that a negative PERC rule result was indicative of a moderate decrease in the probability that someone with clinically suspected PE had a confirmed PE (negative likelihood ratio = 0.21 [95% CI 0.14 to 0.30]). The sensitivity of PERC was 0.95 (95% CI 0.91 to 0.98) and the specificity was 0.23 (95% CI 0.12 to 0.37).
A sensitivity analysis, excluding the two diagnostic accuracy studies at high risk of bias, had very similar results.
The cluster RCT found no difference in mortality, diagnostic strategy failure, and major bleeding events when PERC was used at the start of the diagnostic pathway to rule out PE compared with when it was not. The median length of emergency department stay was significantly shorter with the PERC rule (median difference 37 minutes shorter, interquartile range 4 minutes to 68 minutes shorter).
The results above, along with evidence from the guideline economic model, supported a weak recommendation to consider using the PERC rule when there is a low clinical suspicion of PE to decide which low-risk patients would benefit from further testing.
The guideline committee agreed that, based on the populations in the included studies, a low risk of PE could be defined as an estimated probability of PE <15% based on clinical assessment and the possibility of an alternative diagnosis.
Patients with intermediate or high risk of PE do not require application of the PERC rule as the result will not influence the need for further tests.
Wells score
If a non-pregnant patient with suspected PE is haemodynamically stable, use the Wells (or Geneva) score to assess the clinical probability of PE and to guide investigations.[67][76][77]
The Wells score is a clinical decision tool developed to help guide clinicians’ evaluation of haemodynamically stable patients with suspected acute PE.[98] [ Pulmonary Embolism Wells Score Opens in new window ]
The two-level Wells score categorises patients as ‘PE likely’ (Wells score >4) or ‘PE unlikely’ (Wells score ≤4).[67][76][77]
A simplified version is also available, under which each criterion scores a single point. A total score of 0-1 is categorised as ‘PE unlikely’ and a score of ≥2 is categorised as ‘PE likely’.
An earlier three-level iteration of the Wells score (where patients are categorised into low, moderate, or high clinical probabilities of PE) has been used but the two-level score is generally preferred.[67]
Criterion | Original Wells score (points) |
|---|---|
Clinical signs of deep vein thrombosis (DVT) (minimum of leg swelling and pain with palpation of deep veins) | 3 |
Alternative diagnosis is less likely than PE | 3 |
Previous PE or DVT | 1.5 |
Heart rate >100 bpm | 1.5 |
Surgery (last 4 weeks) or immobilisation for >3 days | 1.5 |
Haemoptysis | 1 |
Active cancer | 1 |
Practical tip
Wells score is commonly used in the UK but an alternative called the Geneva score is also available.
Choose which score to use based on local protocols.
Do not use Wells or Geneva scores in pregnancy. See Suspected PE in pregnancy below.
PE likely (Wells score >4)
Give urgent anticoagulation to any patient with a Wells score >4 (unless contraindicated) while awaiting investigation with definitive imaging (CTPA if available).[67]
If anticoagulation is contraindicated these patients will require close monitoring to observe for signs of cardiorespiratory deterioration. Prioritise these patients for urgent investigation, which may include an urgent echocardiography to assess right ventricular function as well as CTPA to confirm or exclude a PE.
Do not perform D-dimer testing if a patient’s Wells score puts them in the ‘PE likely’ category as imaging is warranted regardless of a negative D-dimer value.[67][74]
PE unlikely (Wells score ≤4)
Request D-dimer testing for all patients with a Wells score ≤4.[76]
If the D-dimer result will not be available within 4 hours, offer anticoagulation (unless contraindicated).[76]
Wells versus Geneva scores
Wells and Geneva scores have similar sensitivity and specificity.
In a systematic review PE was confirmed in 12% of patients classified as ‘PE unlikely’ and in 50% of patients classified as ‘PE likely’.[99]
Neither one of Wells or Geneva score has been shown to be superior to the other. However, the Geneva score is based solely on objective clinical terms and may be more reproducible whereas Wells includes a subjective judgement of whether an alternative diagnosis is less likely than PE.[67]
Sensitivity of the Wells and Geneva scores ranged from 88% to 96% and specificity from 48% to 53% when each clinical decision tool was validated in a primary care dataset.[100]
Laboratory tests
D-dimer
Non-pregnant patients
Use a clinical probability score to determine whether a patient needs D-dimer testing.
Request D-dimer testing in any haemodynamically stable patient whose Wells (or Geneva) score categorises them as ‘PE unlikely’.An alternative approach is to use D-dimer adjusted to age for patients aged over 50 years.[67][74][76] Follow local protocols.
Offer anticoagulation (unless contraindicated) if the D-dimer result cannot be obtained within 4 hours.[76]
Do not use D-dimer testing in patients whose Wells (or Geneva) score categorises them as ‘PE likely’ as a negative result will not remove the need for further imaging.[74]
A normal plasma D-dimer level (typically <500 ng/mL, but check the local threshold) safely excludes PE in patients with an unlikely pre-test probability of PE and no further investigation is required.[67][74][76] The risk of PE within 3 months is <1% in these patients.[101]
If a patient has a raised D-dimer level, start anticoagulation (unless contraindicated) and arrange computed tomographic pulmonary angiography (CTPA) to confirm or exclude PE (unless a concurrent DVT is suspected).[67][74][76]
D-dimer testing is highly sensitive (>95%) in patients in whom pre-test probability suggests that PE is unlikely.[74] However, its specificity is low and may decline with increasing patient age.[74] Use of an age-adjusted D-dimer level may improve the performance of D-dimer testing in patients aged over 50 years.[67][76]
Evidence: Age-adjusted D-dimer
Age-adjusted D-dimer thresholds increase specificity.
A meta-analysis of 13 studies and 12,497 patients without high pre-test clinical probability of PE found that the use of age-adjusted D-dimer cut-offs for patients aged over 50 years (age × 10 ng/mL) maintained a sensitivity for PE above 97% while significantly increasing specificity.[102]
In patients with cancer, using an age-adjusted D-dimer cut-off doubled the proportion of patients in whom PE could be excluded by clinical decision rule and D-dimer, without the need for imaging.[103]
Pregnant patients
Request D-dimer testing in all pregnant patients with suspected PE; use the D-dimer result in combination with the YEARS criteria to assess the clinical probability of PE. The YEARS criteria are:[67][79][80]
Clinical signs of DVT
Haemoptysis
PE is the most likely diagnosis.
PE can be safely ruled out if:[79][80]
None of the YEARS criteria are met and the D-dimer level is <1000 ng/mL
≥1 of the YEARS criteria are met and the D-dimer level is <500 ng/mL.
Start anticoagulation (unless contraindicated) and arrange definitive imaging to confirm or exclude PE (unless a concurrent deep vein thrombosis [DVT] is suspected) if:[79][80]
None of the YEARS criteria are met and the D-dimer level is ≥1000 ng/mL
≥1 of the YEARS criteria are met and the D-dimer level is ≥500 ng/mL.
Practical tip
Order a lower limb compression venous ultrasound if a concurrent DVT is suspected as this may avoid using CTPA.[79]
Practical tip
Remember that a D-dimer level is a continuous variable.
A low D-dimer does not mean no risk of PE and a slightly higher D-dimer does not mean a step increase in PE risk.
The threshold value (check what value is used by your local laboratory) is the optimum for balancing sensitivity and specificity.
In practice, many people have a raised D-dimer without having a PE.
Full blood count
May indicate thrombocytopenia or anaemia. These patients are at an increased risk of complications from bleeding when taking an anticoagulant.
Heparin therapy can be associated with heparin-induced thrombocytopenia; measure platelet count at baseline and regularly throughout treatment.
May also indicate thrombocythaemia or polycythaemia, which increase the risk of venous thromboembolism (VTE).
Urea and electrolytes
Check baseline kidney function. Doses of some anticoagulants (e.g., low molecular weight heparin, fondaparinux, apixaban, rivaroxaban, dabigatran, edoxaban) may need to be adjusted in patients with abnormal kidney function.[76]
Kidney function results can also be used to guide investigations. For example, using CTPA in patients with severe abnormal kidney function can risk contrast-induced nephropathy.
Coagulation studies
Order international normalised ratio (INR), prothrombin time (PT), and activated partial thromboplastin time (aPTT). These are needed to establish baseline values before starting anticoagulation.
Also aids decisions about the safety and choice of initial anticoagulation.[76]
Liver function tests (LFTs)
Abnormal LFTs can influence the choice of anticoagulation.
Arterial blood gas (ABG)
Do not perform an ABG if the oxygen saturations are within normal range on room air.
Consider ABG in patients who have low oxygen saturations. However, be aware that it is of very limited diagnostic utility, either alone or in combination with other clinical variables, in suspected PE.[104]
Evidence: Role of ABG
ABG has a limited role in diagnosing PE.
A PaO 2 <80 mmHg (10.7 kPa), a PaCO 2 <36 mmHg (4.6 kPa), or an abnormal alveolar-arterial gradient (A–aO 2) are not predictive of PE in patients suspected of having PE.[104] [ A-a Gradient Opens in new window ]
In patients with suspected acute PE with normal ABG results, PE could not be excluded in 38% of those without cardiopulmonary disease or in 14% of those who had pre-existing cardiopulmonary disease.[105]
How to obtain an arterial blood sample from the radial artery.
Cardiac biomarkers
The cardiac biomarkers high-sensitivity troponin (either I or T), N-terminal pro-B-type natriuretic peptide (NT-proBNP), or B-type natriuretic peptide (BNP) are not used in diagnosing PE.[106]
They can be used in combination with risk stratification scoring (the full Pulmonary Embolism Severity Index score [PESI], or its simplified sPESI version) to aid prognostic assessment of patients with confirmed or highly suspected PE, particularly to guide decisions about early discharge for low-risk patients.[67]
Measure a cardiac biomarker in a patient with a confirmed or highly suspected PE (positive D-dimer or Wells score >4) if:[67][106]
Risk stratification puts the patient in the intermediate-risk group (PESI III-IV or sPESI ≥1)[67]
OR
Risk stratification puts the patient in the low-risk group (PESI I-II or sPESI 0), AND all of the following criteria are met:
Incidental right ventricular (RV) dysfunction was identified on CTPA or echocardiography performed as part of the diagnostic work-up
AND
The patient is considered potentially suitable for outpatient management[106]
Do not use routinely to risk stratify patients.
Seek senior review if there is an incidental finding of an elevated troponin in a patient with a low-risk PE (PESI I or II/sPESI 0).
An alternative cause for elevated troponin should be considered.[106]
For more detail on risk stratification, see Management recommendations.
Evidence: Cardiac biomarkers
Elevated cardiac biomarkers predict a worse outcome.
Elevated plasma troponin levels on admission have been reported to be associated with a worse prognosis.
A meta-analysis covering a total of 1985 patients showed elevated cardiac troponin I or T concentrations in approximately 50% of the patients with acute PE. Elevated troponin concentrations were associated with higher mortality both in unselected patients and haemodynamically stable patients.[107]
Use of age-adjusted high-sensitivity troponin T cut-off values (≥14 picograms/mL for patients aged <75 years and ≥45 picograms/mL for those aged ≥75 years) may further improve the negative predictive value of this biomarker.[108]
Low levels of BNP or NT-proBNP can identify patients with a favourable short-term outcome based on their high negative predictive value.[109]
The plasma levels of BNP and NT-proBNP reflect the severity of haemodynamic compromise and (presumed) RV dysfunction in acute PE. Right ventricular pressure overload is associated with increased myocardial stretch, which leads to the release of BNP or NT-proBNP and therefore raised plasma levels.[67]
A meta-analysis found that 51% of 1132 unselected patients with acute PE had elevated BNP or NT-proBNP concentrations on admission. These patients had a 10% risk of early death and a 23% risk of an adverse outcome.[110]
Imaging
Chest x-ray (CXR)
A CXR is not diagnostic of PE but may be useful to support the diagnosis and is important to rule out other causes.
It is usually normal in a patient with a PE but an abnormal CXR does not rule out PE.
In a patient with PE, it may show:[76]
Atelectasis
Pleural effusion
Elevation of a hemidiaphragm.
Practical tip
Pulmonary infarction secondary to PE can be difficult to distinguish from pneumonia on CXR.
ECG
An ECG is not diagnostic of PE but can be useful to support the diagnosis of PE or rule out other causes.
A normal ECG does not rule out a PE.[76]
ECG signs that may be present in a patient with PE include:[67][74][111][112]
Normal sinus rhythm
Sinus tachycardia
New right bundle branch block (complete or incomplete)
QR pattern in V1
S1Q3T3 pattern
T wave inversion in V1-V4
ST segment migration in V1 through V4
Atrial arrhythmias, most frequently atrial fibrillation
Non-specific ST-segment and T-wave abnormalities
Right axis deviation
P pulmonale.
Evidence: ECG
Some ECG signs are predictors of a poor outcome.
A systematic review and meta-analysis looked at the value of using ECG in predicting clinical deterioration and mortality in acute PE.
It found that ECG signs that were good predictors of a poor outcome included S1Q3T3, complete right bundle branch block, T-wave inversion, right axis deviation, and atrial fibrillation.[111]
How to record an ECG. Demonstrates placement of chest and limb electrodes.
Computed tomographic pulmonary angiography (CTPA)
CTPA is the preferred investigation for definitive confirmation of PE.
Diagnosis is confirmed by direct visualisation of a thrombus in a pulmonary artery, which appears as a partial or complete intraluminal filling defect.
CTPA is indicated in:
Patients with suspected PE who are haemodynamically unstable at presentation (provided CTPA is immediately available and the patient is well enough to have it - in practice this is often not the case and echocardiography is used instead)
Patients who are classified as ‘PE likely’ based on Wells (or Geneva) score
Patients who are classified as ‘PE unlikely’ based on Wells or Geneva score but who have a positive D-dimer test[76]
Patients who are pregnant with suspected PE and have an abnormal initial chest x-ray.[67]
Avoid CTPA if possible in younger patients, especially if PE can be ruled out by other non-invasive methods with less radiation exposure.[94]
PE does not need to be confirmed on CTPA prior to discharge in low-risk patients (Pulmonary Embolism Severity Index score [PESI] I-II or simplified PESI [sPESI] 0) who are suitable for outpatient management but it should be performed within 24 hours (if available).[106]
Evidence: CTPA risks
CTPA must be used judiciously to ensure benefits outweigh risks.
Radiation from CT is considered to be a risk factor for cancer.
In one study, a cohort of children with CT exposure was found to have a significantly higher incidence of leukaemia and brain tumours later in life.[74]
However, CT technology has evolved, and several techniques can now reduce radiation exposure without compromising image quality, particularly in pregnant women.[113][114]
Both maternal and fetal radiation exposure are low using modern imaging techniques; fetal radiation doses are well below the threshold associated with fetal radiation complications and the effect on maternal cancer risk is negligible (lifetime cancer risk is reportedly increased by a factor of 1.0003 to 1.0007).[113][114][115][116][117][118][119][120]
Therefore, do not avoid CTPA on the grounds of maternal cancer risk.[67]
The contrast dye used in CTPA for the evaluation of PE may cause nephropathy.
In a prospective study, 174 patients underwent CTPA, which demonstrated acute PE in 7%. Some 12% developed contrast-induced nephropathy including one patient with acute PE. Development of contrast-induced nephropathy was associated with an increased risk of severe abnormal kidney function or established kidney failure and death within the subsequent 45 days.[121]
Repeated imaging is common, which may magnify these risks.
In one study performed at a large academic centre, at least one third of emergency department patients who had CTPA for the evaluation of PE underwent another CT for the same reason within 5 years.[122]
More widespread use of CTPA may lead to overdiagnosis of subsegmental PEs that are often of little clinical significance.
The diagnostic value and clinical significance of subsegmental defects identified on CTPA remains hotly debated.[67][123]
Evidence suggests that there has been minimal or no associated change in patient outcomes despite the increased use of CTPA. It is thought that this is due to increased identification of less severe PEs (such as subsegmental PEs) that would not have previously been diagnosed and that may not be clinically relevant.[123][124][125][126]
There is a lack of evidence to support decisions on whether or not to treat patients who have incidental PE detected by CTPA, although they probably should be treated if they have cancer and a proximal clot.[67][123][127]
Potentially inappropriate overuse of CTPA results in increased risks to patients as well as higher costs.[74]
Evidence: CTPA predictive value
The predictive value of CTPA depends on the pre-test probability of PE.
The PIOPED II trial highlighted the influence of clinical probability on the predictive value of CTPA.[128]
The negative predictive value for PE of a negative CTPA was dependent on pre-test clinical probability of PE as assessed by the Wells score (96% for those with a low probability pre-test Wells score and 89% for those with an intermediate probability score, but only 60% for those with a high probability Wells score - based on the three-level Wells Score)
Conversely the positive predictive value of a positive CTPA was high (92% to 96%) in patients with an intermediate or high probability pre-test Wells score but only 58% in those with a low probability pre-test score.
Therefore, clinicians should be particularly cautious in case of discrepancy between clinical judgement and the CTPA result. However a normal CTPA excludes a PE in a patient whose pre-test Wells (or Geneva) score categorised them as PE unlikely.[67]
Echocardiography
Use bedside echocardiography for haemodynamically unstable patients with suspected PE who cannot have CTPA (e.g., if CTPA is not immediately available or the patient is too unwell to be moved).
Echocardiography can also identify important alternative causes of shock, such as pericardial tamponade, acute valvular dysfunction, severe global or regional left ventricular (LV) dysfunction, aortic dissection, or hypovolaemia.
Do not use echocardiography routinely in haemodynamically stable patients with suspected PE.
Findings in PE include:[67]
Abnormal RV ejection pattern (so-called ‘60-60 sign’)
Reduced contractility of the RV free wall compared with the RV apex (‘McConnell sign’)[67]
RV dilatation and hypokinesis
RV diameter/LV diameter >0.9
Interventricular septal flattening and paradoxical leftward septal motion
Presence of tricuspid regurgitation
Presence of pulmonary hypertension (peak tricuspid jet velocity >2.6 m/s and loss of respirophasic inferior vena cava collapse)
Mobile right heart thrombi can be detected by transthoracic or transoesophageal echocardiography (or by CT angiography) in less than 4% of unselected patients with PE. This finding essentially confirms the diagnosis of PE and is associated with RV dysfunction and high early mortality.[67]
Practical tip
A negative echocardiography does not exclude PE.[67]
The reported negative predictive value for PE is 40% to 50%.
Assessment of RV function on echocardiography is not obligatory for the identification of low-risk patients who are suitable for outpatient management.[106]
However, if it has been performed as part of the diagnostic work-up for PE and shows evidence of RV dysfunction, consider measuring a cardiac biomarker to further risk stratify the patient.
Lower limb compression venous ultrasound
Use lower limb compression venous ultrasound to investigate patients suspected of having a concurrent deep vein thrombosis (DVT).
Finding a proximal DVT in patients suspected of having PE is considered sufficient to start anticoagulant treatment without further investigation.[67]
Evidence: Ultrasound
Lower limb ultrasound has high sensitivity/specificity for DVT.
Lower limb compression venous ultrasound has a sensitivity >90% and a specificity of approximately 95% for symptomatic DVT and shows a DVT in 30% to 50% of patients with PE.[67]
Ventilation/perfusion (V/Q) scan
A V/Q scan is being increasingly used as an alternative to CTPA, particularly for patients who have a contraindication to CTPA (such as abnormal kidney function or contrast allergy).[67][74][76][129]
V/Q may be preferred over CTPA in some patient groups to avoid unnecessary radiation, particularly in younger patients in whom CTPA may raise the lifetime risk of cancer.[67][74] It may also be used in pregnant patients who have a normal initial chest x-ray.
A V/Q SPECT (single-photon emission computed tomography) scan is commonly used; a V/Q planar scan is an alternative if this is not available.[76]
Practical tip
A V/Q scan is of limited use in patients with significant underlying lung disease, left ventricular failure, or congestive cardiac failure.[67]
Evidence: Ruling out PE with a V/Q scan
For patients with suspected PE who are unable to have CTPA, guidelines recommend a V/Q SPECT (single-photon emission computed tomography) scan or V/Q planar scan as an alternative; however, the evidence is limited.
The UK National Institute for Health and Care Excellence (NICE) 2020 venous thromboembolic diseases guideline recommends a CTPA scan as first-line imaging in people with suspected PE and a Wells score >4.[76] For people with an allergy to contrast media, with severe abnormal kidney function, or at a high risk from irradiation, NICE recommends assessing the suitability of a V/Q SPECT or a V/Q planar scan (if V/Q SPECT is not available) as an alternative.
NICE identified one randomised controlled trial (n=1417) comparing V/Q scanning with CTPA in patients in whom VTE had initially been excluded.[76][130]
There was a possible clinically important decrease in mortality in patients who received a CTPA scan compared with V/Q scan (RR 0.62 [95% CI 0.34 to 1.11], absolute effect 19 fewer per 1000 [from 32 fewer to 5 more], moderate-quality evidence as assessed by GRADE).
However, there was no difference in symptomatic PE or proximal DVT events (RR 0.36 [95% CI 0.07 to 1.79], absolute effect 6 fewer per 1000 [from 9 fewer to 8 more], GRADE low).
Five diagnostic studies were identified for V/Q scans; one of these studies assessed the diagnostic accuracy of both V/Q planar lung scintigraphy and V/Q SPECT.[76]
Sensitivity and specificity for planar V/Q scans ranged from 41% to 100% and 72% to 97%, respectively. However, some studies had high numbers of non-diagnostic or indeterminate scans that were excluded from the study analysis, making planar V/Q seem more effective (GRADE very low).
One small study (n=41) calculated sensitivity and specificity of V/Q SPECT as 100% and 87%, respectively (GRADE very low). However, five indeterminate scans were not accounted for in the analysis so the accuracy may have been overestimated.[131]
The 2019 European Society of Cardiology (ESC) guideline also states that CTPA is the imaging of choice with V/Q planar scans or V/Q SPECT as an alternative.[67] The ESC notes that the evidence for V/Q SPECT is limited because:
Most studies are retrospective
Some studies include SPECT itself in the reference standard
Diagnostic criteria for PE vary between studies
The optimal scanning technique has not been validated.
PE is the leading cause of maternal mortality in developed countries.[67][78]
There is a fourfold increased risk of venous thromboembolism (VTE) throughout pregnancy compared with non-pregnant women and this is relatively equal across all three trimesters.
This risk rises sharply in the postnatal period and is up to 60 times higher in the 3 months after delivery, compared with the risk in non-pregnant women.
Symptoms and signs of PE may be less specific in pregnant women.[74]
Be cautious in interpreting breathlessness as it is a common symptom in pregnancy.[67]
If you suspect PE in a pregnant patient, seek advice from a senior clinician.
Do not use the Wells (or Geneva) score in pregnancy - they are not reliable predictors of the probability of PE. Instead, use the YEARS criteria in combination with a D-dimer level to determine whether further definitive imaging is necessary.[79][80]
Use lower limb compression ultrasound if concurrent deep vein thrombosis (DVT) is suspected. Some centres now advocate bilateral lower limb ultrasound even if there are no clinical signs of DVT.
If no concurrent DVT is suspected, order a chest x-ray (CXR).[67] If the CXR is:
Abnormal: computed tomographic pulmonary angiography (CTPA) is the first-line option for definitive diagnosis of PE, after other causes of chest symptoms have been considered[67]
Normal: use either CTPA or V/Q scan based on local availability and protocols.[67]
Evidence: Clinical probability scores in pregnancy
The YEARS algorithm (YEARS criteria combined with a D-dimer level) can safely exclude PE in pregnant patients without the need for definitive imaging.
Previously, advanced imaging was the only validated method of excluding PE in pregnant patients.[80]
However, evidence has emerged that shows that risk stratification can be used to safely exclude PE in pregnant patients without definitive imaging.[79][80]
One study evaluated the Righini algorithm, which comprised the revised Geneva score, leg ultrasound, CTPA and/or V-Q scan, and D-dimer level. None of the 367 patients in whom PE was excluded had symptomatic VTE during the 3-month follow-up period, and PE was excluded in 11.6% of patients without imaging, all of whom would presumably have received imaging otherwise.[132]
The YEARS algorithm compares favourably with the Righini algorithm. In a study of 498 patients, only one patient in whom PE was excluded was diagnosed with DVT at 3-month follow-up. No patients were diagnosed with PE during this period. The YEARS algorithm was more efficient than the Righini algorithm, and imaging was avoided in 65% of patients enrolled in the first trimester, 46% in the second trimester, and 32% in the third trimester.[80]
In a more recent post-hoc analysis of 371 patients, the YEARS algorithm safely excluded PE in all patients who met the PE exclusion criteria.[79]
Data are lacking on the validity of Wells score in pregnancy.
A retrospective series of 125 pregnant women who were referred for CTPA showed that no patient with an initial Wells score <6 had a PE.[133]
An unprovoked PE is a PE in a patient who had no pre-existing, transient provoking risk factor in the prior 3 months.[76]
Provoking risk factors include: surgery; trauma; significant immobility (bedbound, unable to walk unaided, or likely to spend a substantial proportion of the day in bed or in a chair); pregnancy or puerperium; use of oral contraceptive/hormone replacement therapy).
An unprovoked PE may be suggestive of an underlying condition so further investigations are sometimes warranted.
Undiagnosed cancer
In any patient diagnosed with unprovoked PE who is not known to have cancer:[76]
Review medical history
Review baseline blood tests including full blood count, kidney and hepatic function, prothrombin time (PT) and activated partial thromboplastin time (APTT)
Offer a physical examination.
Do not offer further investigations for cancer for patients with an unprovoked PE unless they have relevant clinical symptoms or signs.[76]
Evidence: Cancer screening
The evidence supporting screening for undiagnosed cancer after unprovoked venous thromboembolism is inconclusive.
A screening strategy that was proposed in the SOMIT trial included pelvic and abdominal CT combined with mammography and sputum cytology. This was recommended as the most effective and least harmful approach for patients.
However, no 5-year survival benefit was found when this approach was compared with basic clinical evaluation.[134][135]
Thrombophilia testing
Consider testing for hereditary thrombophilia in patients who don't have an identifiable risk factor and have a first degree relative who has had a VTE, if it is planned to stop anticoagulation.[76]
Do not routinely offer thrombophilia testing to first-degree relatives of people with a history of PE and thrombophilia.[76][136]
Consider testing for antiphospholipid antibodies in patients who have had an unprovoked PE if it is planned to stop anticoagulation treatment.[76]
Practical tip
Be aware that tests for hereditary thrombophilia and antiphospholipid antibodies can be affected by anticoagulation; specialist advice may be needed.[76]
Biomarkers
Evidence has shown that certain biomarkers may be of use when assessing prognosis in PE. These include: heart-type fatty acid-binding protein (H-FABP), lactate, neutrophil gelatinase-associated lipocalin, cystatin C, creatinine, and vasopressin. However, they are not widely used in clinical practice.[67]
These biomarkers have been combined with clinical parameters to form prognostic scoring tools (e.g., the Bova score), although the implications for patient management remain unclear.[67]
Point-of-care D-dimer testing
Point-of-care D-dimer testing may be increasingly used if laboratory facilities are not immediately available (e.g., in the community to avoid sending a patient to hospital and particularly in remote areas where access to health care is limited). However, point-of-care assays have a lower sensitivity and negative predictive value compared with laboratory-based D-dimer tests. Therefore, point-of-care D-dimer testing should only be used in patients with a low pre-test probability.[67][76]
D-dimer testing adjusted to clinical probability
Evidence suggests that D-dimer testing adjusted to clinical probability is effective in non-pregnant patients in addition to pregnant patients, but this is not in widespread clinical use.
A prospective management trial used the YEARS criteria in combination with D-dimer. Computed tomographic pulmonary angiography was avoided in 48% of patients, compared with 34% if the Wells criteria and fixed D-dimer threshold of 500 ng/mL had been used.[67] Symptomatic venous thromboembolism was diagnosed in 0.61% (95% CI 0.36% to 0.96%) of patients who had PE initially ruled out at baseline and were left untreated during the 3 month follow-up period.[67]
Magnetic resonance angiography (MRA):
The hypothesis is that a negative MRA combined with the absence of proximal deep vein thrombosis on lower limb compression ultrasound may safely rule out clinically significant PE.[67]
This is being tested in a multicentre outcome study.[137]
MRA has shown promise but is not yet ready for clinical practice due to its low sensitivity, high proportion of inconclusive scans, and low availability in most emergency settings.
Use of this content is subject to our disclaimer