Recommendations
Urgent
Suspect new-onset atrial fibrillation (AF) if the patient has an irregularly irregular pulse with or without any one of:[3][32][33]
Palpitations - the cardinal symptom
Dyspnoea
Chest pain
Fatigue
Dizziness
Polyuria
Syncope.
Perform manual pulse palpation and conduct an immediate ECG. Take an entire 12-lead ECG recording.[1][3] A single-lead ECG tracing of ≥30 seconds is also diagnostic.[1]
Irregularity of pulse rate can be difficult to discern during rapid pulse rates.[3]
Diagnostic ECG features of AF:[1]
No discernible or distinct P wave activity
AND
Irregularly irregular ventricular rate (R-R intervals; where atrioventricular conduction is not impaired).
Urgently assess for:[1]
Features of life-threatening haemodynamic instability
Symptoms of acute myocardial ischaemia
Suspected or confirmed serious precipitating illness requiring hospital care
AF alongside a pre-excitation syndrome such as Wolff-Parkinson-White syndrome
Signs or symptoms of acute stroke.
These patients may need emergency electrical cardioversion.[1][3] See Emergency management of haemodynamically unstable patients under Management recommendations.
Seek advice from a cardiology/arrhythmia specialist or a senior colleague if the patient is haemodynamically compromised, or if you are uncertain how to proceed.
Key Recommendations
Be aware that 50% to 87% of people with AF are initially asymptomatic.[1]
In every acute presentation of AF, assess:[1][3]
For signs of a stroke
Stroke risk using the CHA2DS2-VASc score[34] [ Atrial Fibrillation CHA(2)DS(2)-VASc Score for Stroke Risk Opens in new window ]
Bleeding risk using either the ORBIT score or the HAS-BLED score[3][34][35][36] [ ORBIT Bleeding Risk Score Opens in new window ] [ HAS-BLED Bleeding Risk Score Opens in new window ]
Risk factors, comorbidities, and sequelae of the presentation
Duration of onset of AF
Presence of haemodynamic instability
Presence of pulmonary oedema
Presence of acute ischaemia
Whether there is an underlying cause that is reversible.
Use these factors to guide management. For more detail, see Management recommendations.
Look for symptoms that are caused by the fast and irregular ventricular rate, including:
Palpitations[1][3][32][33][37]
The cardinal symptom
Might be described by the patient as 'heart racing' or 'fluttering in the chest'
Can last for minutes to hours; occuring on rest or on activity
Breathlessness/dyspnoea[1][3][32][33][37]
May be secondary to obesity or underlying:
Coronary artery disease
Left ventricular dysfunction with or without acute pulmonary oedema. See Acute heart failure
Pulmonary disease
May be directly related to the rapid and irregular ventricular rate
Chest pain/tightness/discomfort[1][3][32][37]
Often due to the rapid and irregular ventricular rate; ensure you exclude rate-related ischaemia
Anxiety[37]
Cognitive impairment[33]
Owing to rapid and irregular ventricular rate
Polyuria[33]
Due to both a tachycardia-induced diuresis and natriuresis
Occasionally: pre-syncope and syncope[1][3][37]
As a result of rapid and irregular ventricular rate leading to reduced cardiac output and subsequent reduced cerebral perfusion.
Pay special attention to features that suggest acute haemodynamic instability:[1]
Syncope
Acute pulmonary oedema
Ongoing myocardial ischaemia
Symptomatic hypotension
Cardiogenic shock.
These patients may need emergency electrical cardioversion. See Emergency management of haemodynamically unstable patients under Management recommendations.
Seek advice from a cardiology/arrhythmia specialist or a senior colleague if the patient is haemodynamically compromised, or if you are uncertain how to proceed.
Practical tip
Although acute atrial fibrillation will usually present as tachycardia due to a fast ventricular rate, in practice bradycardia is sometimes seen in older people with age-related cardiac conduction disease.
Bear in mind that the first presentation of AF may be with a stroke, heart failure, or cardiac ischaemia.[38] See Ischaemic stroke, Stroke due to spontaneous intracerebral haemorrhage, Acute heart failure, and Myocarditis.
Look for focal neurological deficits such as hemiplegia or dysphasia.
Practical tip
Between 50% and 87% of people with AF are initially asymptomatic.[1] In these groups, AF tends to be diagnosed incidentally during routine physical examinations, pre-operative assessments, or population surveys.[39] Asymptomatic clinical AF has been independently associated with increased risk of stroke and mortality compared with symptomatic AF.
AF-related heart failure
Be aware that AF can present with heart failure, which can be in a patient with:
Heart failure decompensating due to incident AF, or
AF developing heart failure following chronically poor heart rate control; sometimes termed tachycardiomyopathy, tachycardia-induced cardiomyopathy, or arrhythmia-induced cardiomyopathy.
Practical tip
Perform a transthoracic echocardiogram within 48 hours of admission in people presenting with new suspected heart failure in the absence of a recent echocardiogram.[40] In practice, refer confirmed heart failure to a specialist.
Tachycardiomyopathy
Have a high index of clinical suspicion in an unexplained heart failure presentation.
Patients with tachycardiomyopathy typically present with acute AF and unexplained symptoms and signs of heart failure.[41] They will often have recent-onset 'silent' AF in the preceding weeks or months, with an uncontrolled ventricular rate that causes cardiac decompensation.[42][43]
Tachycardiomyopathy is under-recognised.
Presentation is often late following heart failure symptoms.
Tachycardiomyopathy is potentially reversible.
Take a complete history, including:[1][3]
Time since symptom onset
Type and intensity of symptoms
Relevant risk factors and medical history
Possible precipitants and assessment of concomitant conditions.
Time since symptom onset
Crucially, aim to ascertain when symptoms began, paying particular attention to whether they began within the last 24 hours, the last 48 hours, or more.
This will affect your approach to management. See Better symptom management under Management recommendations.
Based on the latest evidence, 2020 guidelines from the European Society of Cardiology recommend that a safe time-frame for cardioversion without a strict need for post-cardioversion anticoagulation is 24 hours from onset.[1] Conversely, the UK National Institute for Health and Care Excellence recommends a time-frame of 48 hours from onset in its 2014 guideline.[3]
The duration of AF (<48 hours or ≥48 hours) will also affect options for rhythm control, and whether the patient has early, delayed, or elective cardioversion.[1]
In practice, determining an exact time from symptom onset to first medical contact can be difficult. Overall, there is poor correlation between symptoms and AF.
The patient will generally only give an approximate idea of when their symptoms began.
They may also have ignored their symptoms for some time until they felt they could no longer tolerate the symptoms. In some cases, the patient may have had asymptomatic AF.
Practical tip
If it is unclear when symptoms started, err on the side of caution and manage as if it has been more than 48 hours since onset.
Type and intensity of symptoms
Remember that palpitations are the cardinal symptom.[3][32]
Other associated symptoms may occur in conjunction with palpitations. See Presentation above.
Bear in mind that the patient may describe less severe or shorter duration symptom episodes preceding the more severe episode that has precipitated their call for medical assistance.
Ask about chest pain and shortness of breath.[32]
Use the modified European Heart Rhythm Association (EHRA) symptom scale to quantify the patient’s symptom status before, and after initiation of, treatment.[1]
The EHRA symptom scale evaluates the effect of six symptoms (palpitations, fatigue, dizziness, dyspnoea, chest pain, and anxiety) during AF on the patient’s daily activity, ranging from none to symptom frequency or severity that leads to a discontinuation of daily activities.[44]
Relevant risk factors and medical history
Specifically consider:
Advancing age[14]
Prevalence of AF: 0.5% in people aged 50 to 59 years; 8.8% in people aged 80 to 89 years[15]
Hypertension
Heart failure
Approximately 40% of patients admitted with heart failure and reduced ejection fraction and 50% of patients admitted with heart failure and preserved ejection fraction have AF on ECG[16]
Diabetes
Associated with a 50% increase in risk of AF[13]
Obstructive sleep apnoea
Obesity
Associated with a 50% increase in the risk of AF[19]
Coronary artery disease
Prevalence in AF: 41%[21]
Valve disease (in particular, mitral valve disease and rheumatic heart disease)
Other cardiac disease (e.g., valvular, congenital, cardiomyopathy)[22]
Alcohol use[24]
Previous cardiothoracic interventions
Previous atrial arrhythmias
Previous stroke/transient ischaemic attack
Hyperthyroidism
Athletic levels of physical activity
Limited data suggest that athletes may have a higher risk of developing AF.[27]
Possible precipitants
Prioritise treating any precipitating factors (e.g., an underlying medical illness) you identify, as the AF will only respond to treatment once the precipitant is under control.[1] Focus on excluding:
Active infection; sepsis[1][45][46]
See Sepsis in adults
Dehydration
Pericarditis
See Pericarditis
Myocarditis
See Myocarditis
Recent surgical intervention[1]
Pulmonary conditions (e.g., pneumonia, pulmonary embolism, acute pulmonary oedema)[1][32]
Metabolic disturbances (e.g., hypomagnesaemia, acid-base disturbance)
Thyroid dysfunction
Can also be hypothyroidism
Illicit drug use
Phaeochromocytoma (rare).
Practical tip
If the patient has delirium, dementia, post-stroke aphasia, or impaired communication for any reason, check for acute urinary retention as a precipitant of tachyarrhythmia.
The patient may report alcohol as a trigger for an AF episode.
Regular light‐moderate alcohol consumption (<14 units/week) without binge drinking is not associated with an increased risk of AF.[23]
However, regular heavier alcohol consumption (>14 units/week) is associated with an increased risk of AF.[23]
Practical tip
Use the mnemonic PIRATES to help remember common causes:
Pulmonary (pneumonia; pulmonary embolism; pulmonary disease)
Ischaemia/infarction/idiopathic
Rheumatic valvular disease (mitral stenosis or regurgitation)
Alcohol/anaemia/age/autonomic tone
Thyroid disease (hyperthyroidism)
Elevated blood pressure/electrolytes/endocarditis
Sepsis/sleep apnoea/surgery
Your priorities are to:
Determine the duration of symptoms[1]
Look for concomitant conditions that may be the underlying cause of the AF[1][32]
Identify AF-related sequelae including stroke, systemic thromboembolism, and heart failure.[1]
Perform manual pulse palpation of the radial artery to assess for the presence of an irregular pulse in any patient presenting with any one of the following:[3]
Palpitations
Dyspnoea/breathlessness
Chest discomfort
Syncope/dizziness
Stroke/transient ischaemic attack.
Bear in mind that an irregular pulse on palpation alone is characteristic, but not diagnostic, of AF. ECG is the gold-standard for diagnosis of AF.[1] See Investigations below.
Practical tip
Given that the diagnosis of AF can only be confirmed with an ECG, alternative conditions are difficult to differentiate from the history or examination alone. Common differentials include atrial flutter and atrial tachycardia, which are discernible on ECG as a supraventricular tachycardia that is a more organised rhythm than AF. For more information, see Differentials.
Auscultate the heart and lungs.[32] Check for:
Variable intensity of first heart sound[47]
Crackles[48]
If bilateral: suggests heart failure
If unilateral: might suggest pneumonia
Cardiac wheeze
Secondary to heart failure ± pulmonary oedema.
Your initial examination should also check:
Airway patency
Breathing, including oxygen saturations
To determine whether there is a significant respiratory component
Circulation
Heart rate
Blood pressure[32]
Jugular venous pressure (JVP)
Look for a raised JVP and absent ‘a’ waves
Disability; check consciousness level
Use the Glasgow Coma Scale or AVPU [alert, verbal, pain, unresponsive] scale. [ Glasgow Coma Scale Opens in new window ]
Evidence: Diagnostic accuracy of pulse palpation to detect AF
Pulse palpation for an irregular pulse is sensitive in the detection of new-onset atrial fibrillation in people aged 65 years and over, although the number of false positives is high.
In its 2006 guideline on AF the UK National Institute for Health and Care Excellence (NICE) conducted an evidence review into how sensitive an irregular pulse is as a screening test for AF.[3] It identified two studies, both in a UK primary care setting in people over 65 years old with a diagnosis of AF confirmed by ECG.[49][50]
Pulse palpation for any irregularity was sensitive for detecting AF (93% to 100% depending on age and sex), and it was unlikely that AF was present if the pulse was normal (negative predictive value >96%).[50]
Specificity fell to 71% in people over 75 years of age; however, the positive predictive value (PPV - the proportion of positive results that are true positives) was greater in those over 75 years old due to the increased prevalence of AF in this population (PPV women 65 to 74: 12%; women ≥75: 23%; men 65 to 74: 8%; men ≥75: 14%).[50]
In a nurse-led screening programme, if the criterion of any pulse irregularity was used, sensitivity was 91% and specificity was 74%. This was reversed if continuous irregularity was used (sensitivity 54%, specificity 98%).[49]
The number needed to screen to identify one additional patient with AF was 31 (95% CI 23 to 50).
NICE also mentioned the Screening for Atrial Fibrillation in the Elderly (SAFE) study, a randomised controlled trial and cost-effectiveness study published in October 2005 (outside of the date limits of its systematic literature search).[51][52]
This confirmed the diagnostic accuracy of pulse palpation (sensitivity 87.2%, specificity 81.3%; false negatives 2% and false positives 70%).
The economic model showed that opportunistic case-detection for AF is more cost-effective than systematic screening with fewer ischaemic strokes and a greater proportion of diagnosed AF.
NICE did not feel this evidence required updating when it updated the guideline in 2021.
Practical tip
In practice, an accurate assessment of pulse rate and regularity may require palpation of the carotids or auscultation. Palpation of other sites may cause decreased perfusion, which potentially influences the findings. In addition, an irregular pulse can be difficult to discern if the pulse rate is very fast or slow.
Seek specialist input from an arrhythmia specialist or cardiologist for any patient with:
Features of life-threatening haemodynamic instability
Symptomatic hypotension (systolic blood pressure <90 mmHg) or other signs of shock
Chest pain or evidence of myocardial ischaemia on ECG
Signs of reduced cerebral perfusion (reduced conscious level/Glasgow Coma Scale, syncope)
Signs of heart failure
Persistent tachycardia
Severe angina or worsening left ventricular function
Transient ischaemic attack or stroke
Suspected heart failure
A pre-excitation syndrome such as Wolff-Parkinson-White syndrome[53]
Suspected underlying structural heart disease who is young
Uncontrolled rate
Symptomatic bradycardia not amenable to reduction of rate control agents See Management section.
Liaise with a senior colleague if cardiology advice is not available (e.g., it is out of hours).
These groups may need urgent electrical cardioversion. See Emergency management of haemodynamically unstable patients under Management recommendations.
Anticoagulation does not routinely require specialist input (and should be initiated immediately/early in all suitable patients).[1]
All patients
ECG
Perform an immediate ECG in any patient with an irregular pulse, whether symptomatic or not, who you suspect has AF.[1][3][32] Take an entire 12-lead ECG recording.[1][3] A single-lead ECG tracing of ≥30 seconds is also diagnostic.[1]
ECG is the gold-standard for diagnosis of AF.[1] An irregular pulse on palpation alone is characteristic, but not diagnostic, of AF.
Look for the hallmark ECG features of AF:[1]
No discernible or distinct P wave activity (chaotic baseline)
AND
Irregularly irregular ventricular rate (R-R intervals; where atrioventricular conduction is not impaired)
[Figure caption and citation for the preceding image starts]: Atrial fibrillationFrom the collections of Arti N. Shah and Bharat K. Kantharia [Citation ends].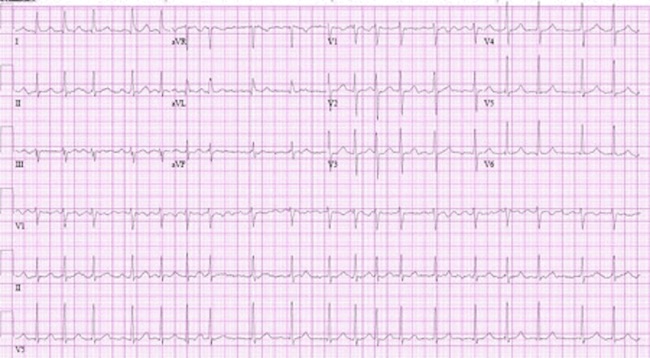
Practical tip
Don’t trust the computer. Computerised ECG interpretation is prone to error, particularly in patients who are continuously or intermittently paced.[54][55] Ensure any automated interpretation is verified in person by a skilled reader of ECGs. Ventricular/atrial ectopics are key differentials for an irregularly irregular pulse.
As well as informing diagnosis, use ECG to screen for:
Conduction defects
If there is AF and atrioventricular conduction block, a slow regular ventricular escape rhythm is usually present with no distinct P waves
Ischaemia or structural heart disease
ST–T-wave changes may be present; caused by rate-related ischaemia, digoxin therapy, or structural heart disease (such as hypertrophic obstructive cardiomyopathy)
Underlying structural heart disease
Primary electrical disorders such as Brugada pattern (right bundle-branch block with ST-segment elevation in the V1-3 leads) or a pre-excitation syndrome such as Wolff-Parkinson-White syndrome. See Wolff-Parkinson-White syndrome.
Practical tip
It’s easy to miss patients with AF and complete heart block. Look out for the key ECG features:[56]
No distinct P-waves; just fibrillary waves of AF
Regular ventricular rhythm
The wider the QRS of the ventricular escape rhythm the less reliable the escape mechanism.
These patients will often need a pacemaker (if they are not taking rate-limiting drugs).
How to record an ECG. Demonstrates placement of chest and limb electrodes.
Laboratory work-up
Send blood samples for routine haematological and biochemical analysis as soon as possible. Do not delay management by waiting for these test results; start treatment straight away (urgent cardioversion may be required).
Ensure your blood panel for all patients includes:
To detect non-cardiac factors precipitating AF (e.g., anaemia or infection)
Clotting profile[32]
Take as a baseline to identify any patient with an underlying coagulation defect and inform management with anticoagulants
Including electrolytes, urea, creatinine
To exclude renal impairment, hypokalaemia, hyperkalaemia, and hypomagnesaemia
Chronic kidney disease is a general cardiac risk factor and a specific risk factor for AF[57]
Estimate creatinine clearance using the Cockroft-Gault equation; this will help inform anticoagulant dosing [ Creatinine Clearance Estimate by Cockcroft-Gault Equation Opens in new window ]
To exclude thyrotoxicosis (suppressed thyroid-stimulating hormone with elevated free T4 and/or T3).
Chest x-ray
Request a chest x-ray as routine for all cardiac presentations, particularly if you suspect lung pathology.
Transthoracic echocardiography
In line with 2020 guidelines from the European Society of Cardiology, request transthoracic echocardiography (TTE) in all patients to check for:[1]
Left ventricular size and function
Left atrium size
Valvular disease
Right ventricular size and function.
Bear in mind that 2014 guidelines from the National Institute for Health and Care Excellence in the UK recommend only requesting a TTE in specific circumstances, particularly for people with AF:
For whom a baseline echocardiogram is important for long-term management[3]
In practice, this will be where there is no obvious reversible non-cardiac cause that is unlikely to have other cardiac consequences (e.g., infection or thyrotoxicosis)
For whom you are considering a rhythm-control strategy that includes cardioversion (electrical or pharmacological)[3]
In whom there is a high risk of, or you suspect, underlying structural/functional heart disease (such as heart failure or heart murmur) that influences their subsequent management (e.g., choice of anti-arrhythmic drug)[3]
In whom refinement of clinical risk stratification for antithrombotic therapy is needed.[3]
Practical tip
Perform a transthoracic echocardiogram within 48 hours of admission in people presenting with new suspected heart failure in the absence of a recent echocardiogram.[40] In practice, refer confirmed heart failure to a specialist.
Selected patients
Laboratory work-up
Request for selected patients:
Cardiac troponin[1]
High-sensitivity cardiac troponin T may be elevated due to prolonged tachycardia or post DC cardioversion but not at the levels usually seen with myocardial infarction and acute coronary syndromes
Arterial blood gases
To exclude hypoxia
C-reactive protein[1]
B-natriuretic peptide (BNP)/N-terminal prohormone B-natriuretic peptide (NT-pro-BNP)[1]
Liver function tests (LFTs)[32]
Use to exclude the presence of a multisystem disorder affecting the liver
Also use to guide choice of appropriate anti-arrhythmic drugs and to monitor anti-arrhythmic therapy. For example, amiodarone is contraindicated in the presence of liver dysfunction; amiodarone treatment should be discontinued when LFTs show abnormalities[58]
Erythrocyte sedimentation rate.
Transoesophageal echocardiography (TOE)
Discuss arranging a TOE, if available, with a cardiologist in people with atrial fibrillation when:[1][3]
TTE demonstrates an abnormality (such as valvular heart disease) that warrants further specific assessment
TTE is technically difficult and/or of questionable quality and there is a need to exclude cardiac abnormalities
You are considering TOE-guided cardioversion.
Practical tip
TOE may not be readily available in the usual settings of care (e.g., accident and emergency department or general practice office). Therefore, in practice, base management decisions on the likely duration of arrhythmia rather than the presence or absence of intracardiac clot on echocardiographic imaging.
[Figure caption and citation for the preceding image starts]: Transoesophageal echocardiogram showing left atrial appendage clot. LA, left atrium; LAA, left atrial appendage; LV, left ventricleFrom the collection of Dr Bharat Kantharia [Citation ends].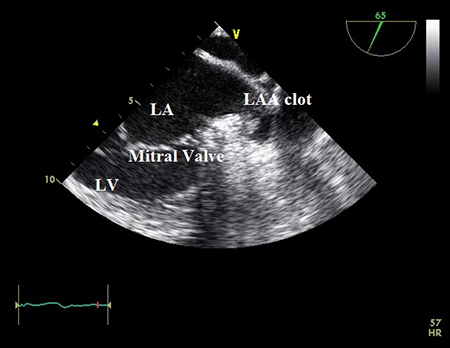
Other investigations
Consider, based on individual patient presentations:
Inpatient telemetry or 24-hour holter monitor[1][32]
To assess heart-rate control and/or bradycardia, conduction disturbances
To help titrate drugs used for rate or rhythm control
Coronary angiography (CT or conventional) or stress testing[1]
If you suspect significant coronary artery disease from the patient’s history and risk factor profile
Late gadolinium contrast-enhanced cardiac magnetic resonance (CMR)[1]
If, after TOE/TTE, you suspect structural heart disease or cardiomyopathic process, late gadolinium contrast-enhanced CMR can help guide management decisions
Brain imaging: CT or MRI[1]
In patients with AF and signs of cerebral ischaemia or stroke, imaging the brain can help detect stroke and guide management decisions regarding acute management and long-term anticoagulation
Computed tomographic pulmonary angiography (CTPA)
If AF is secondary to pulmonary embolism. CTPA is the preferred investigation for definitive confirmation of pulmonary embolism in most patients.[60][61] See Pulmonary embolism.
Use of this content is subject to our disclaimer