Recommendations
Urgent
Put out an immediate cardiac arrest call for any patient with suspected tension pneumothorax and give high-flow oxygen. Immediate decompression is required; do not wait for imaging results to confirm the diagnosis.
Unless the tension pneumothorax is secondary to trauma, insert a large-bore cannula into the pleural space through the second intercostal space in the mid-clavicular line or the fourth or fifth intercostal space in the mid-axillary line. A ‘hiss’ of air confirms the diagnosis.[40][47]
Use a standard safety cannula. Do not use a blood control (closed system) cannula for tension pneumothorax decompression. NHS England. National Patient Safety Alert – Blood control safety cannula and needle thoracostomy for tension pneumothorax. Apr 2020 Opens in new window
Insert a chest drain immediately after needle decompression. Only insert a chest drain yourself if you have had adequate training and are appropriately supervised.[65]
If the tension pneumothorax is secondary to trauma, use open thoracostomy for decompression if the expertise is available.[47]
The Advanced Trauma Life Support guideline now recommends using the fourth or fifth intercostal space in the mid-axillary line as first-line if needle decompression is required.[66]
Key Recommendations
[Figure caption and citation for the preceding image starts]: BTS clinical pathway decision tree for pneumothorax. COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; OPD, outpatient department; PSP, primary spontaneous pneumothorax; SSP, secondary spontaneous pneumothoraxRoberts ME et al. Thorax 2023; 78: 1143-56; used with permission [Citation ends].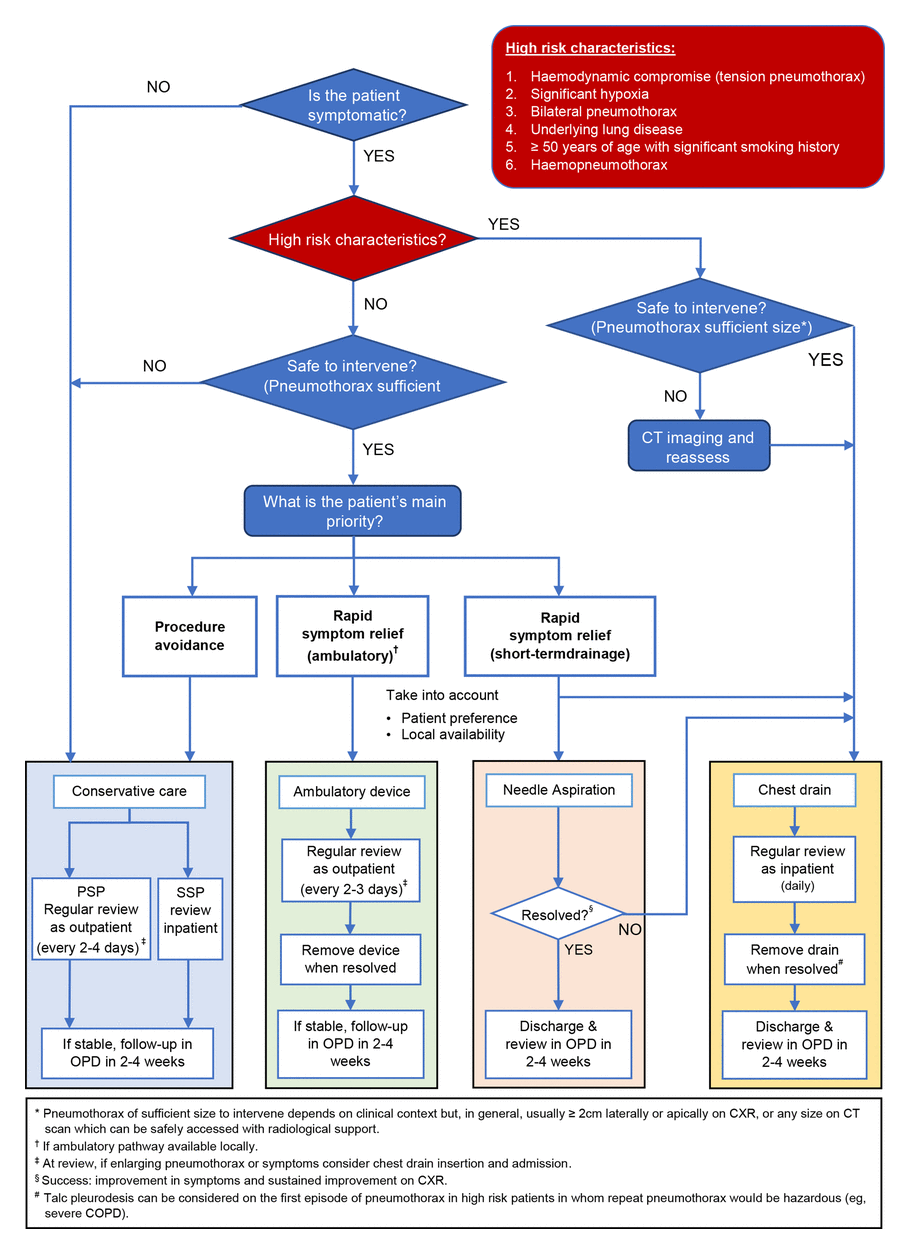
Manage pneumothorax according to the type (e.g., primary, secondary, traumatic) as well as the clinical status of the patient. Refer the patient to the respiratory team within 24 hours if they are being admitted.[8][17][47]
The British Thoracic Society (BTS) now advises that the size of a pneumothorax is no longer an indication for invasive management; however, it does dictate the safety of conducting an intervention.[17]
When managing spontaneous pneumothorax, according to the 2023 BTS guidelines:[17]
Minimally symptomatic patients (i.e., no significant pain or breathlessness and no physiological compromise) and asymptomatic patients can be managed conservatively
Consider ambulatory management in adults with access to good support and follow-up facilities
Review these patients regularly (i.e., every 2-4 days until stable)
Consider needle aspiration or tube drainage in symptomatic patients with a large primary spontaneous pneumothorax (PSP) or in patients otherwise not deemed suitable for conservative management
To prevent a recurrent secondary spontaneous pneumothorax in adults (e.g., in patients with severe COPD who significantly decompensated in the presence of a pneumothorax), consider chemical pleurodesis
Consider thoracic surgery at initial presentation if recurrence prevention is deemed important (e.g., in patients presenting with tension pneumothorax, or those in high-risk occupations).
Give high-flow oxygen and target oxygen saturations of close to 100% for all patients who are being admitted for observation who have not had a chest drain inserted, unless they are at risk of hypercapnic (type II) respiratory failure.[42]
Give supplemental oxygen to all other patients if required to maintain oxygen saturations of 94% to 98% (or 88% to 92% if they are at risk of hypercapnic [type II] respiratory failure).[42]
If considering discharge, ensure the patient has adequate follow-up in 2-4 weeks. Give them verbal and written information to return if they develop further breathlessness, as well as lifestyle advice to prevent recurrence.[17]
If surgical intervention is required:[17]
For general management, consider video-assisted thoracoscopy access for surgical pleurodesis
Consider thoracotomy access and surgical pleurodesis for the lowest level of recurrence risk required for specific (e.g., high-risk) occupations
Consider surgical pleurodesis and/or bullectomy for the treatment of spontaneous pneumothorax.
Give high-flow oxygen and target oxygen saturations of close to 100% in all patients (unless they are at risk of hypercapnic [type II] respiratory failure) with:[42]
Tension pneumothorax
Pneumothorax that requires admission for observation without insertion of a chest drain.
Give supplemental oxygen to all other patients if required to maintain oxygen saturations of 94% to 98% (or 88% to 92% if they are at risk of hypercapnic [type II] respiratory failure).[42]
Guidelines on oxygen therapy often recommend an upper target saturation of 96%. However, pneumothorax is a specific scenario where higher target oxygen saturations are advised; once there is clinico-radiological evidence of resolution of a pneumothorax, supplemental oxygen should not be needed unless there is underlying lung pathology such as COPD, asthma, or pneumonia.[42][67]
Evidence: High-flow oxygen
It is widely accepted that oxygen therapy increases the resolution rate of spontaneous pneumothorax; however, studies were based on small populations.[68][69][70]
One small case series study examined the rate of resolution of pneumothorax in 8 patients who received room air or high concentration oxygen delivered by a partial rebreathing mask.[69]
Compared with a rate of resolution of 1.25% per day when the patients breathed room air, the rate increased to 4.2% per day when breathing a high concentration of inspired oxygen.
In a larger study, the rate of absorption was measured in 12 patients breathing air (group 1), and in 10 patients during periods breathing air and other periods breathing oxygen at 6 L/minute (group 2).[68]
While breathing air, the mean rate of absorption in group 2 (4.8 cm 2/24 hours) was similar to that in group 1.
While breathing oxygen, however, the rate of absorption in group 2 increased to a mean of 17.9 cm2/24 hours (P <0.01).
The calculated time for full re-expansion for the patients in group 2 varied from 8 to 45 days (mean 22.5 days) with continuous inhalation of air, and from 3 to 8 days (mean 5.4 days) with daily oxygen therapy.
Other authors have highlighted the need to consider potential adverse effects of oxygen.[70]
The use of high concentration oxygen can shorten the duration of treatment before pneumothorax resolution.[68][69]
Tension pneumothorax
Put out an immediate cardiac arrest call for any patient with suspected tension pneumothorax and give high-flow oxygen. Immediate decompression is required; do not wait for confirmation of the tension pneumothorax on imaging.[17]
If the tension pneumothorax is not secondary to trauma, insert a large-bore cannula into the pleural space through the second intercostal space in the mid-clavicular line or the fourth or fifth intercostal space in the mid-axillary line. A ‘hiss’ of air confirms the diagnosis.[40][47]
Use a standard safety cannula. Do not use a blood control (closed system) cannula for tension pneumothorax decompression. NHS England. National Patient Safety Alert – Blood control safety cannula and needle thoracostomy for tension pneumothorax. Apr 2020 Opens in new window
Insert a chest drain immediately after needle decompression.[65]
If the tension pneumothorax is secondary to trauma, use open thoracostomy for decompression if the expertise is available.[47]
The Advanced Trauma Life Support guideline now recommends using the fourth or fifth intercostal space in the mid-axillary line as first-line if needle decompression is required.[66]
How to decompress a tension pneumothorax. Demonstrates insertion of a large-bore intravenous cannula into the fourth intercostal space in an adult.
Practical tip
A standard cannula for needle decompression may be too short if used in the second intercostal space, mid-clavicular line, in some patients. The chest wall may be less deep in the fourth or fifth intercostal space, mid-axillary line, and therefore this site is an alternative for decompression; it can also be used as a site for chest drain insertion if there is initial failure with needle decompression. However, the reverse may be true in practice for patients with a high body mass index.
Bilateral pneumothorax
Insert a chest drain; start with the larger of the two pneumothoraces and involve senior support.
Spontaneous pneumothorax
Manage spontaneous pneumothorax according to whether a patient has high-risk characteristics and factoring in the patient’s main priority (procedure avoidance or rapid symptom relief), as well as the clinical status of the patient.[17]
Practical tip
The distinction between primary and secondary spontaneous pneumothorax is becoming increasingly blurred in practice. Seek senior or specialist advice if you are in any doubt about which type of pneumothorax your patient has.
In theory, a patient with any underlying respiratory diagnosis should be considered to have a secondary, rather than primary, spontaneous pneumothorax. Most cases of secondary spontaneous pneumothorax are in patients with COPD.[2]
However, in practice, a patient with a history of only very mild respiratory disease might be managed as a primary spontaneous pneumothorax - for example, a patient with mild, intermittent asthma with no exacerbation of symptoms at presentation with the pneumothorax.
[Figure caption and citation for the preceding image starts]: BTS clinical pathway decision tree for pneumothorax. COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; OPD, outpatient department; PSP, primary spontaneous pneumothorax; SSP, secondary spontaneous pneumothoraxRoberts ME et al. Thorax 2023; 78: 1143-56; used with permission [Citation ends].
The British Thoracic Society (BTS) now advises that the size of a pneumothorax is no longer an indication for invasive management; however, it does dictate the safety of conducting an intervention.[17]
Consider conservative management in asymptomatic patients with normal observations.[17][74]
In symptomatic patients, assess whether they have any high-risk characteristics, including:[17]
Significant hypoxia
Haemodynamic compromise
Bilateral pneumothorax
Underlying lung disease
Age ≥50 years with significant smoking history
Haemopneumothorax.
In the absence of high-risk characteristics, management can be guided by the patient’s main priority:
Consider conservative care if procedure avoidance is a priority
Consider ambulatory management with purpose-made devices or a Heimlich one-way valve (where facilities are available), OR needle aspiration where rapid symptom relief is a priority. Ensure pneumothorax is sufficient size to intervene (usually ≥2 cm laterally or apically on CXR).
Review patients undergoing conservative care regularly.
Patients with a PSP can be reviewed regularly as an outpatient (i.e., every 2-4 days until stable).
Patients with a secondary spontaneous pneumothorax (SSP) should be reviewed as inpatients.
In patients where needle aspiration fails, or in symptomatic patients with high-risk characteristics, insert a chest drain. Ensure that the pneumothorax is of sufficient size for this intervention: the BTS states that this will depend on the clinical context but is generally a pneumothorax ≥2 cm in size laterally or apically on CXR, or any size on CT scan that can be safely accessed with radiological support.[17]
Correct clotting abnormalities (INR ≥1.5 and platelets ≤50 x 109/L) before inserting a chest drain in patients who are not critically unwell.[52][53] Follow up patients who have had an intervention in 2-4 weeks as an outpatient.
Consider thoracic surgery at initial presentation if recurrence prevention is deemed important (e.g., in patients presenting with tension pneumothorax, or those in high-risk occupations).[17]
Consider talc pleurodesis on the first episode of pneumothorax in high-risk patients in whom repeat pneumothorax would be hazardous (e.g., in patients with severe COPD).[17]
Practical tip
Obtain written consent prior to insertion of a chest drain, unless the patient is critically unwell.[65]
Ensure adequate analgesia prior to chest drain insertion in stable patients and use plenty of local anaesthetic during insertion as it is a very painful procedure.[43]
A small-bore chest drain (≤14F) is generally recommended for spontaneous pneumothorax in non-ventilated patients.[52][65]
The patient is usually positioned at 45° to 60° with their ipsilateral arm above their head. However, for a traumatic pneumothorax, the chest drain is normally inserted while the patient is supine.[65]
The drain should be inserted aseptically; the Seldinger technique is most commonly used.[52][65]
How to insert an intercostal (chest) drain using the Seldinger technique. Video demonstrates: how to identify a safe site for insertion; use of an introducer needle, guidewire, dilators, and intercostal drain; how to confirm drain position; and post-procedure care.
How to insert an intercostal (chest) drain using the open technique. Video demonstrates: tube selection, how to identify the site for drain insertion, how to make the correct incision, how to insert the intercostal drain, how to secure the drain, and post-procedure care.
Following insertion of a chest drain it is essential to:[81]
Check the underwater seal oscillates during respiration
Order a repeat chest x-ray to confirm the position of the drain and the degree of lung re-expansion, and to exclude any complications
Advise the patient to keep the underwater bottle upright and below the drain insertion site
Ensure regular analgesia is prescribed while the chest drain is in place.
Traumatic (non-tension) pneumothorax
Traumatic pneumothorax should be managed by a thoracic surgeon; management will depend on the size of the pneumothorax and the clinical status of the patient. This may include a chest drain or thoracotomy.[12]
Never leave a patient with a penetrating chest wound or open pneumothorax unattended as tension pneumothorax may develop. Cover the wound with a simple occlusive dressing and observe closely.[43][47]
Give a dose of prophylactic antibiotics if a chest drain is being inserted to decrease the risk of empyema and pneumonia.[82] Check local protocols.
Refer all patients who are being admitted to the respiratory team within 24 hours of admission.[17]
Chest drain management is best delivered by nurses with specialist expertise.
Observe the patient for at least 24 hours if they require admission but do not need a chest drain.
Monitor for complications of chest drain insertion, which occur in 8% to 20% of procedures.[52] Visceral injury is the most serious complication, but other more common complications include:[17]
Pain
Intrapleural or wound infection
Drain dislodgement or blockage
Surgical emphysema.
Practical tip
Use of suction too early after chest drain insertion may cause re-expansion pulmonary oedema, especially in the case of a primary spontaneous pneumothorax that may have been present for more than a few days.
Order a chest x-ray if the chest drain stops bubbling to determine whether this is due to blockage or malpositioning of the drain or resolution of the pneumothorax.[81]
If the drain has stopped bubbling but is still swinging, this implies resolution of the pneumothorax with a patent drain.
If the drain is not swinging or bubbling, the drain may be blocked. Try flushing the drain as this may clear the blockage.
Only remove a chest drain based on senior or specialist advice. The decision to remove a chest drain depends on the clinical situation.[52] Usually, chest drains should be removed only when there is a re-expansion of the lung and no clinical evidence of an air leak.[39]
Prepare for removal of a chest drain by ensuring any drain on suction is placed on 'water seal' and remove any sutures or dressings that are holding it in place.
Remove the drain quickly at the end of expiration during a Valsalva manoeuvre and place a sterile dressing immediately over the insertion site.
Cover the insertion site with an occlusive dressing. Only suture the insertion site if a large-bore (≥18F) drain has been used.
Order a chest x-ray to ensure the lung remains fully re-expanded. This is normally performed several hours after chest drain removal.
Early discussion with a thoracic surgeon can help balance the risks of ongoing air leak and potentially unnecessary surgical procedures, particularly in patients who meet the following criteria:[83]
Persistent air leak
Failure of the lung to re-expand
Presentation with tension pneumothorax
Patients in high-risk occupations
First secondary pneumothorax associated with significant physiological compromise.
Also seek advice from a thoracic surgeon for patients with:[17]
Second ipsilateral pneumothorax
First contralateral pneumothorax
Synchronous bilateral spontaneous pneumothorax
Spontaneous haemothorax
Pregnancy.
The main aims of surgery are to repair a persistent air leak and prevent recurrence.[17]
Thoracic surgery for pneumothorax can be broadly divided into two different types:
Surgical pleurodesis to obliterate the pleural space via an inflammatory symphysis of the visceral and parietal pleura to prevent the accumulation of air within that space and prevent any future episodes of pneumothorax.
This can be achieved through several different methods intra-operatively, including pleural abrasion, partial pleurectomy, and talc poudrage.
Resection of lung parenchyma or a ‘bullectomy’ to remove the suspected source of the current air leak and prevent future potential sources of air leak.
This requires a form of wedge resection using stapler equipment, and can also include the use of a ‘sealant’ (such as glue and a mesh) to further fortify the site of lung resection.
Options include open thoracotomy or video-assisted thoracoscopic surgery (VATS).[17]
Consider VATS surgical pleurodesis in the general management of pneumothorax.
Consider thoracotomy access and surgical pleurodesis for the lowest level of recurrence risk required for specific (e.g., high-risk) occupations. Pneumothorax recurrence rates and the need for further procedures appear to be slightly increased following VATS when compared with thoracotomy.[17]
Evidence: Surgical management
Evidence comparing the two surgical operations for pneumothorax, surgical pleurodesis and resection of lung parenchyma, shows virtually no difference between risks and benefits of the two treatments.[17]
A literature search identified 10 studies relevant to the review, including 2 randomised controlled trials and 8 retrospective cohort studies.[84]
There was no difference in pneumothorax recurrence, length of hospital stay, the need for further treatment (surgery, chest drain, or conservative management), duration of air leak, complications, or mortality.
Pain and breathlessness and quality of life were also reviewed; however, there was insufficient evidence to comment on these.
The British Thoracic Society guideline on pleural disease recommends that both operations, surgical pleurodesis and resection of lung parenchyma, should be considered for the treatment of spontaneous pneumothorax in adults.[17]
If the patient has a spontaneous pneumothorax and a persistent air leak, but is unable or unwilling to undergo surgery, options include:[17]
Autologous blood pleurodesis
Endobronchial therapies.
Evidence: Management of persistent air leak in patients who are unsuitable for surgery
There are several treatment options available, including application of thoracic suction, converting to larger-bore chest drain, blood patch or chemical pleurodesis, endobronchial valves, or thoracic surgery. Indications for surgery include a persistent air leak (despite 5-7 days of chest tube drainage) or failure of lung re-expansion.
The British Thoracic Society guideline on pleural disease recommends considering autologous blood pleurodesis or endobronchial therapies as options for patients with spontaneous pneumothorax who have persistent air leaks and are unfit for surgery.[17]
Due to a lack of evidence, only the above two strategies were reviewed. In the review it was found that:
Length of hospital stay appeared to be shorter following autologous blood pleurodesis treatment, regardless of delivery method, when compared with chest drainage alone[17]
There was no evidence to suggest that the application of suction is not beneficial to treat pneumothorax and persistent air leak[17]
Limited evidence suggested that endobronchial therapies may have had the potential to treat pneumothorax and persistent air leak.
There is currently insufficient evidence to make any recommendations on the best treatment method for pneumothorax and persistent air leaks.
Catamenial pneumothorax
Management includes a combination of surgical intervention and hormonal manipulation to suppress ovulation and menstruation.[17][85]
Pneumothorax ex vacuo
Management depends on the underlying cause and should aim to alleviate the endobronchial obstruction. For example, bronchoscopy may be used if the pneumothorax is secondary to endobronchial obstruction with lobar or whole lung collapse.[86]
Chest drain is not generally recommended, especially in asymptomatic patients.[87]
HIV
Patients with HIV infection and pneumothorax require early insertion of a chest drain and surgical referral as well as treatment for HIV and any associated Pneumocystis jirovecii infection.[88]
Cystic fibrosis
Secondary spontaneous pneumothorax is a common complication of cystic fibrosis and is associated with a poor prognosis, the median survival being 30 months.[89] A small asymptomatic pneumothorax can be observed or aspirated, whereas larger pneumothoraces require a chest drain.[17] Chest tube drainage alone has a recurrence rate of 50%, but with interventions such as pleurectomy, pleural abrasion and pleurodesis recurrence can be reduced.[90][91]
Discharge practices vary according to the management plan, but usually all patients will require follow up in 2-4 weeks in an outpatient department (OPD) on discharge. If patients have received:[17]
Conservative care - review regularly every 2-4 days as an outpatient. Once stable can discharge with follow-up in OPD.
Ambulatory care - regularly review every 2-3 days as an outpatient. Once resolved can remove device and once stable can discharge with follow-up in OPD.
Needle aspiration - discharge once resolved with follow-up in OPD.
Chest drain - will be treated as an inpatient. Remove drain once resolved and discharge with follow-up in OPD.
Evidence: Conservative management
Evidence supports the view that patients with primary spontaneous pneumothoraces without treatment, and those with larger primary spontaneous pneumothoraces after aspiration, may not need hospitalisation as the likelihood of complications is low.[93]
The British Thoracic Society guideline on pleural disease states that minimally symptomatic patients (i.e., patients without breathlessness, significant chest pain, or physiological compromise) and asymptomatic patients may be treated conservatively and managed as outpatients regardless of the size of the pneumothorax, assuming they have ready access to medical care if required.[17]
Underpinning evidence comes from a randomised controlled trial and retrospective cohort studies of patients treated conservatively.[46][94][95][96]
Evidence found that length of hospital stay, risk of pneumothorax recurrence, and rate of complications appear to be reduced when these patients are managed conservatively compared with intercostal drainage.[17]
For larger primary spontaneous pneumothoraces after aspiration, outpatient treatment is possible in many cases.[93]
A Cochrane systematic review including 6 randomised controlled trials and a total of 435 patients with primary spontaneous pneumothoraces requiring drainage (either symptomatic or >20%) compared simple aspiration with chest tube drainage.[97]
Around 58% of the aspiration group achieved immediate (near) complete lung expansion (6 studies; moderate-quality evidence as assessed using GRADE).
Around 37% required admission after the procedure (3 studies; very low quality evidence as assessed using GRADE).
Fewer adverse events occurred when patients were treated by simple aspiration than by tube drainage, including less perceived pain and lower pain scores, reduced need for thoracoscopic pleurodesis, and fewer technical adverse events. The authors add that this information should be viewed with caution due to the low quality of evidence for this outcome.
The conservative approach and outpatient treatment may be appropriate in patients with primary spontaneous pneumothorax who can easily return to medical care if their symptoms deteriorate.[17]
Give all patients the following written and verbal advice prior to discharge:[17]
They should return as an emergency if they develop further breathlessness
They can return to work and can resume normal physical activity once symptoms have resolved, unless they have a high-risk occupation (e.g., airline pilot)
They should avoid diving permanently, unless a definitive prevention strategy has been performed such as surgical pleurectomy
They should avoid air travel until full resolution of the pneumothorax; resolution must be confirmed on chest x-ray[98]
They should be given smoking cessation advice; smoking influences the risk of recurrence.[17] Smoking cessation reduces this risk fourfold.[8]
Ensure there is adequate follow-up.
All patients require follow-up with a respiratory physician to:[17]
Ensure full resolution of the pneumothorax
Give optimal care of any underlying lung disease
Explain the risk of recurrence and need for possible surgical intervention
Reinforce lifestyle advice on issues such as smoking and air travel.
Organise a follow-up chest x-ray after 2 to 4 weeks to monitor resolution of the pneumothorax for all patients who were managed with observation alone or by needle aspiration.[17]
How to decompress a tension pneumothorax. Demonstrates insertion of a large-bore intravenous cannula into the fourth intercostal space in an adult.
Video demonstrating how to perform a pleural aspiration
How to insert an intercostal (chest) drain using the Seldinger technique. Video demonstrates: how to identify a safe site for insertion; use of an introducer needle, guidewire, dilators, and intercostal drain; how to confirm drain position; and post-procedure care.
How to insert an intercostal (chest) drain using the open technique. Video demonstrates: tube selection, how to identify the site for drain insertion, how to make the correct incision, how to insert the intercostal drain, how to secure the drain, and post-procedure care.
Use of this content is subject to our disclaimer