The diagnosis of primary aldosteronism (PA) should be considered in all patients with hypertension, regardless of severity and plasma potassium level. When present, symptoms suggestive of hypokalaemia (such as muscle weakness, paraesthesias, muscle cramps, nocturia, polyuria, and palpitations) are highly suggestive of PA. However, these symptoms are usually absent, as most patients are normokalaemic.[31]Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004 Mar;89(3):1045-50.
https://academic.oup.com/jcem/article/89/3/1045/2844051
http://www.ncbi.nlm.nih.gov/pubmed/15001583?tool=bestpractice.com
Other symptoms or signs that may be present are usually non-specific and non-contributory to diagnosis. These may include lethargy, difficulty concentrating, and mood disturbances such as irritability, anxiety, and depression.
A critical component of the diagnostic work-up is careful discussion with the patient. Each phase of the diagnostic process should be explained in detail to the patient before a decision is made whether to proceed with it.
Screening
Because only a minority (approximately 20%) of patients with PA are hypokalaemic, measurement of plasma potassium lacks sensitivity as a screening test. However, when hypokalaemia is present (especially when not provoked by the use of diuretics), it serves as a valuable clue towards the presence of this condition.
The aldosterone/renin ratio is the most reliable available screening test, being more specific than renin measurement (levels of which are almost always suppressed) and more sensitive than plasma potassium or aldosterone measurement.[10]Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021 Dec;9(12):876-92.
http://www.ncbi.nlm.nih.gov/pubmed/34798068?tool=bestpractice.com
[28]Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916.
https://academic.oup.com/jcem/article/101/5/1889/2804729
http://www.ncbi.nlm.nih.gov/pubmed/26934393?tool=bestpractice.com
[41]Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020 Oct;38(10):1919-28.
http://www.ncbi.nlm.nih.gov/pubmed/32890264?tool=bestpractice.com
[70]National Comprehensive Cancer Network. NCCN guidelines: neuroendocrine and adrenal tumors [internet publication].
https://www.nccn.org/guidelines/category_1
The ratio becomes elevated before aldosterone or plasma potassium leave their normal ranges.[6]Stowasser M, Gordon RD, Rutherford JC, et al. Diagnosis and management of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2001 Sep;2(3):156-69.
http://journals.sagepub.com/doi/pdf/10.3317/jraas.2001.022
http://www.ncbi.nlm.nih.gov/pubmed/11881117?tool=bestpractice.com
[71]Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511.
http://www.ncbi.nlm.nih.gov/pubmed/9221268?tool=bestpractice.com
[72]Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004 Sep-Oct;14(5):267-76.
http://journals.lww.com/theendocrinologist/Abstract/2004/09000/The_Aldosterone_Renin_Ratio_in_Screening_for.7.aspx
However, false positives and negatives are possible.[10]Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021 Dec;9(12):876-92.
http://www.ncbi.nlm.nih.gov/pubmed/34798068?tool=bestpractice.com
[28]Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916.
https://academic.oup.com/jcem/article/101/5/1889/2804729
http://www.ncbi.nlm.nih.gov/pubmed/26934393?tool=bestpractice.com
[73]Stowasser M, Ahmed AH, Pimenta E, et al. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012 Mar;44(3):170-6.
http://www.ncbi.nlm.nih.gov/pubmed/22147655?tool=bestpractice.com
Dietary salt restriction, concomitant malignant or renovascular hypertension, pregnancy (in which high levels of progesterone antagonise aldosterone action at the mineralocorticoid receptor), and treatment with diuretics (including spironolactone), dihydropyridine calcium channel antagonists, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor antagonists can all lead to false-negative ratios by stimulation of renin secretion.[6]Stowasser M, Gordon RD, Rutherford JC, et al. Diagnosis and management of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2001 Sep;2(3):156-69.
http://journals.sagepub.com/doi/pdf/10.3317/jraas.2001.022
http://www.ncbi.nlm.nih.gov/pubmed/11881117?tool=bestpractice.com
[71]Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511.
http://www.ncbi.nlm.nih.gov/pubmed/9221268?tool=bestpractice.com
[72]Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004 Sep-Oct;14(5):267-76.
http://journals.lww.com/theendocrinologist/Abstract/2004/09000/The_Aldosterone_Renin_Ratio_in_Screening_for.7.aspx
[74]Mulatero P, Rabbia F, Milan A, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002 Dec;40(6):897-902.
http://hyper.ahajournals.org/content/40/6/897.full
http://www.ncbi.nlm.nih.gov/pubmed/12468576?tool=bestpractice.com
[75]Seifarth C, Trenkel S, Schobel H, et al. Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. Clin Endocrinol (Oxf). 2002 Oct;57(4):457-65.
http://www.ncbi.nlm.nih.gov/pubmed/12354127?tool=bestpractice.com
[76]Brown MJ, Hopper RV. Calcium-channel blockade can mask the diagnosis of Conn's syndrome. Postgrad Med J. 1999 Apr;75(882):235-6.
http://www.ncbi.nlm.nih.gov/pubmed/10715768?tool=bestpractice.com
[77]Gordon RD, Tunny TJ. Aldosterone-producing adenoma (A-P-A): effect of pregnancy. Clin Exp Hypertens A. 1982;4(9-10):1685-93.
http://www.ncbi.nlm.nih.gov/pubmed/6754149?tool=bestpractice.com
[78]Stowasser M, Gordon RD, Klemm SA, et al. Renin-aldosterone response to dexamethasone in glucocorticoid-suppressible hyperaldosteronism is altered by coexistent renal artery stenosis. J Clin Endocrinol Metab. 1993 Sep;77(3):800-4.
http://www.ncbi.nlm.nih.gov/pubmed/8396580?tool=bestpractice.com
Because potassium is a powerful chronic regulator of aldosterone secretion, hypokalaemia may also be associated with false-negative ratios.[73]Stowasser M, Ahmed AH, Pimenta E, et al. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012 Mar;44(3):170-6.
http://www.ncbi.nlm.nih.gov/pubmed/22147655?tool=bestpractice.com
Beta-blockers, alpha-methyldopa, clonidine, and non-steroidal anti-inflammatory drugs (NSAIDs) can suppress renin levels and produce false-positives.[6]Stowasser M, Gordon RD, Rutherford JC, et al. Diagnosis and management of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2001 Sep;2(3):156-69.
http://journals.sagepub.com/doi/pdf/10.3317/jraas.2001.022
http://www.ncbi.nlm.nih.gov/pubmed/11881117?tool=bestpractice.com
[10]Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021 Dec;9(12):876-92.
http://www.ncbi.nlm.nih.gov/pubmed/34798068?tool=bestpractice.com
[41]Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020 Oct;38(10):1919-28.
http://www.ncbi.nlm.nih.gov/pubmed/32890264?tool=bestpractice.com
[71]Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511.
http://www.ncbi.nlm.nih.gov/pubmed/9221268?tool=bestpractice.com
[74]Mulatero P, Rabbia F, Milan A, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002 Dec;40(6):897-902.
http://hyper.ahajournals.org/content/40/6/897.full
http://www.ncbi.nlm.nih.gov/pubmed/12468576?tool=bestpractice.com
[79]Ahmed AH, Gordon RD, Taylor P, et al. Effect of atenolol on aldosterone/renin ratio calculated by both plasma renin activity and direct renin concentration in healthy male volunteers. J Clin Endocrinol Metab. 2010 Jul;95(7):3201-6.
https://academic.oup.com/jcem/article/95/7/3201/2596261
http://www.ncbi.nlm.nih.gov/pubmed/20427490?tool=bestpractice.com
False-positives can occur in premenopausal women during the luteal phase of the menstrual cycle, and also in women receiving oestrogen-containing contraceptive agents or hormone replacement therapy, but only when renin is measured as direct active renin concentration and not as plasma renin activity.[80]Ahmed AH, Gordon RD, Taylor PJ, et al. Effect of contraceptives on aldosterone/renin ratio may vary according to the components of contraceptive, renin assay method, and possibly route of administration. J Clin Endocrinol Metab. 2011 Jun;96(6):1797-804.
https://academic.oup.com/jcem/article/96/6/1797/2834422
http://www.ncbi.nlm.nih.gov/pubmed/21411552?tool=bestpractice.com
[81]Ahmed AH, Gordon RD, Taylor PJ, et al. Are women more at risk of false-positive primary aldosteronism screening and unnecessary suppression testing than men? J Clin Endocrinol Metab. 2011 Feb;96(2):E340-6.
https://academic.oup.com/jcem/article/96/2/E340/2709561
http://www.ncbi.nlm.nih.gov/pubmed/20962019?tool=bestpractice.com
[82]Ahmed AH, Gordon RD, Ward G, et al. Effect of combined hormonal replacement therapy on the aldosterone/renin ratio in postmenopausal women. J Clin Endocrinol Metab. 2017 Jul 1;102(7):2329-34.
http://www.ncbi.nlm.nih.gov/pubmed/28379474?tool=bestpractice.com
False-positives may also be seen in patients with impaired renal function (renin production is reduced, whereas any associated hyperkalaemia tends to elevate aldosterone), in advancing age (during which production of renin falls more quickly than that of aldosterone), and in familial hyperkalaemic hypertension, also known as pseudohypoaldosteronism type II or Gordon syndrome.[10]Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021 Dec;9(12):876-92.
http://www.ncbi.nlm.nih.gov/pubmed/34798068?tool=bestpractice.com
[72]Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004 Sep-Oct;14(5):267-76.
http://journals.lww.com/theendocrinologist/Abstract/2004/09000/The_Aldosterone_Renin_Ratio_in_Screening_for.7.aspx
[83]McKenna TJ, Sequeira SJ, Heffernan A, et al. Diagnosis under random conditions of all disorders of the renin-angiotensin-aldosterone axis, including primary aldosteronism. J Clin Endocrinol Metab. 1991 Nov;73(5):952-7.
http://www.ncbi.nlm.nih.gov/pubmed/1939533?tool=bestpractice.com
Treatment with antidepressants of the selective serotonin reuptake inhibitor (SSRI) class lowers the aldosterone/renin ratio, but whether they can cause false-negatives in patients with PA remains uncertain.[84]Ahmed AH, Calvird M, Gordon RD, et al. Effects of two selective serotonin reuptake inhibitor antidepressants, sertraline and escitalopram, on aldosterone/renin ratio in normotensive depressed male patients. J Clin Endocrinol Metab. 2011 Apr;96(4):1039-45.
https://academic.oup.com/jcem/article/96/4/1039/2720852
http://www.ncbi.nlm.nih.gov/pubmed/21289246?tool=bestpractice.com
Diuretics should be discontinued for at least 6 weeks and other interfering medicines for at least 2 (and preferably 4) weeks before measuring the ratio, substituting other medicines that have a lesser effect on results, such as verapamil slow-release (plus or minus hydralazine), prazosin, and moxonidine, in order to maintain hypertension control.[71]Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511.
http://www.ncbi.nlm.nih.gov/pubmed/9221268?tool=bestpractice.com
[72]Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004 Sep-Oct;14(5):267-76.
http://journals.lww.com/theendocrinologist/Abstract/2004/09000/The_Aldosterone_Renin_Ratio_in_Screening_for.7.aspx
[85]Ahmed A, Gordon RD, Ward G, et al. Effect of moxonidine on the aldosterone/renin ratio in healthy male volunteers. J Clin Endocrinol Metab. 2017 Jun 1;102(6):2039-43.
http://www.ncbi.nlm.nih.gov/pubmed/28324033?tool=bestpractice.com
In cases where a potentially interfering medicine cannot be withdrawn, useful information can still be obtained by taking into account its known effects when interpreting the ratio result. For example, an elevated ratio in patients receiving a diuretic, ACE inhibitor, angiotensin receptor blocker, or dihydropyridine calcium blocker would make PA very likely, whereas a normal ratio in the presence of beta-blocker treatment would make the diagnosis very unlikely.
Hypokalaemia should be corrected and the patient should be encouraged to follow a liberal salt diet before ratio measurement. Because of the effects of posture and time of day, sensitivity of the ratio is maximised by collecting blood mid-morning from seated patients who have been upright (sitting, standing, or walking) for 2 to 4 hours.[71]Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511.
http://www.ncbi.nlm.nih.gov/pubmed/9221268?tool=bestpractice.com
[72]Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004 Sep-Oct;14(5):267-76.
http://journals.lww.com/theendocrinologist/Abstract/2004/09000/The_Aldosterone_Renin_Ratio_in_Screening_for.7.aspx
The ratio should be regarded as a screening test only, and should be measured more than once (serially if conditions of sampling, including medicines, are being altered) before deciding whether to go on to a suppression test to definitively confirm or exclude the diagnosis.
Confirmation of diagnosis
Because the aldosterone/renin ratio is not without occasional false-positive results, even under the conditions described above, confirmatory testing is required before the diagnosis of PA can be definitively confirmed or excluded.[10]Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021 Dec;9(12):876-92.
http://www.ncbi.nlm.nih.gov/pubmed/34798068?tool=bestpractice.com
[41]Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020 Oct;38(10):1919-28.
http://www.ncbi.nlm.nih.gov/pubmed/32890264?tool=bestpractice.com
While fludrocortisone suppression testing, in which the aldosterone response during 4 days' administration of oral fludrocortisone and oral salt loading is determined, is widely regarded as the most reliable means of confirming or excluding PA, measurement of plasma aldosterone at the conclusion of an intravenous infusion of 0.9% saline (usually 2 L over 2-4 hours), or 24-hour urinary aldosterone excretion rates following 3 days of oral salt loading are also employed in some centres.[6]Stowasser M, Gordon RD, Rutherford JC, et al. Diagnosis and management of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2001 Sep;2(3):156-69.
http://journals.sagepub.com/doi/pdf/10.3317/jraas.2001.022
http://www.ncbi.nlm.nih.gov/pubmed/11881117?tool=bestpractice.com
[28]Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916.
https://academic.oup.com/jcem/article/101/5/1889/2804729
http://www.ncbi.nlm.nih.gov/pubmed/26934393?tool=bestpractice.com
[72]Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004 Sep-Oct;14(5):267-76.
http://journals.lww.com/theendocrinologist/Abstract/2004/09000/The_Aldosterone_Renin_Ratio_in_Screening_for.7.aspx
[86]Litchfield WR, Dluhy RG. Primary aldosteronism. Endocrinol Metab Clin North Am. 1995 Sep;24(3):593-612.
http://www.ncbi.nlm.nih.gov/pubmed/8575411?tool=bestpractice.com
[87]Holland OB, Brown H, Kuhnert LV, et al. Further evaluation of saline infusion for the diagnosis of primary aldosteronism. Hypertension. 1984 Sep-Oct;6(5):717-23.
http://hyper.ahajournals.org/content/hypertensionaha/6/5/717.full.pdf
http://www.ncbi.nlm.nih.gov/pubmed/6389337?tool=bestpractice.com
[88]Kem DC, Weinberger MH, Mayes DM, et al. Saline suppression of plasma aldosterone in hypertension. Arch Intern Med. 1971 Sep;128(3):380-6.
http://www.ncbi.nlm.nih.gov/pubmed/5093210?tool=bestpractice.com
[89]Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018 Nov 1;103(11):4113-24.
https://academic.oup.com/jcem/article/103/11/4113/5098354
http://www.ncbi.nlm.nih.gov/pubmed/30239841?tool=bestpractice.com
A study involving 100 patients (77 with PA) found saline suppression testing demonstrated better sensitivity for PA when performed in the upright (seated) position compared with the traditional recumbent position.[89]Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018 Nov 1;103(11):4113-24.
https://academic.oup.com/jcem/article/103/11/4113/5098354
http://www.ncbi.nlm.nih.gov/pubmed/30239841?tool=bestpractice.com
Subtype differentiation
If the confirmatory test is positive, further investigations are directed towards determining the subtype of PA (unilateral aldosteronism, mainly caused by aldosterone-producing adenoma; or bilateral adrenal hyperplasia), as the treatment of first choice for each subtype differs.[28]Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916.
https://academic.oup.com/jcem/article/101/5/1889/2804729
http://www.ncbi.nlm.nih.gov/pubmed/26934393?tool=bestpractice.com
Familial hyperaldosteronism type I (FH-I) is rare, but important to diagnose as hypertension is readily controlled by treatment with glucocorticoids.[11]Itcho K, Oki K, Ohno H, et al. Update on genetics of primary adosteronism. Biomedicines. 2021 Apr 10;9(4):409.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8069207
http://www.ncbi.nlm.nih.gov/pubmed/33920271?tool=bestpractice.com
If FH-I is suspected (for example, on the basis of early onset of PA or a family history of early onset hypertension, PA, or stroke), genetic testing of peripheral blood for the hybrid gene should be performed before going on to other tests aimed at subtype differentiation, as a positive genetic test will make them superfluous. Because presence of the hybrid gene is diagnostic for FH-I, testing for it has virtually supplanted the tedious and less reliable biochemical methods of diagnosing this subtype (e.g., demonstration of marked, persistent suppression of plasma aldosterone during several days of dexamethasone administration).[90]Jonsson JR, Klemm SA, Tunny TJ, et al. A new genetic test for familial hyperaldosteronism type I aids in the detection of curable hypertension. Biochem Biophys Res Commun. 1995 Feb 15;207(2):565-71.
http://www.ncbi.nlm.nih.gov/pubmed/7864844?tool=bestpractice.com
[91]Stowasser M, Bachmann AW, Jonsson JR, et al. Clinical, biochemical and genetic approaches to the detection of familial hyperaldosteronism type I. J Hypertens. 1995 Dec;13(12 Pt 2):1610-3.
http://www.ncbi.nlm.nih.gov/pubmed/8903619?tool=bestpractice.com
[92]Mulatero P, Veglio F, Pilon C, et al. Diagnosis of glucocorticoid-remediable aldosteronism in primary aldosteronism: aldosterone response to dexamethasone and long polymerase chain reaction for chimeric gene. J Clin Endocrinol Metab. 1998 Jul;83(7):2573-5.
https://academic.oup.com/jcem/article/83/7/2573/2865742
http://www.ncbi.nlm.nih.gov/pubmed/9661646?tool=bestpractice.com
The great majority of patients with PA, however, will test negative for the hybrid gene, leaving the more difficult task of separating the unilateral tumourous forms from varieties of bilateral adrenal hyperplasia (BAH). In patients with early onset PA who test negative for the hybrid gene, consideration should be given for genetic testing for mutations in CLCN2, KCNJ5, and CACNA1H for diagnosis of FH-II, FH-III, and FH-IV respectively.[41]Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020 Oct;38(10):1919-28.
http://www.ncbi.nlm.nih.gov/pubmed/32890264?tool=bestpractice.com
Adrenal CT scanning is recommended in all patients to confirm subtype and exclude adrenocortical carcinoma.[10]Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021 Dec;9(12):876-92.
http://www.ncbi.nlm.nih.gov/pubmed/34798068?tool=bestpractice.com
[28]Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916.
https://academic.oup.com/jcem/article/101/5/1889/2804729
http://www.ncbi.nlm.nih.gov/pubmed/26934393?tool=bestpractice.com
[41]Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020 Oct;38(10):1919-28.
http://www.ncbi.nlm.nih.gov/pubmed/32890264?tool=bestpractice.com
It is usually able to detect aldosterone-producing carcinomas because of their relatively large size (usually >3 cm) but frequently misses aldosterone-producing adenomas (which have an average size of approximately 1 cm).[9]Mulatero P, Sechi LA, Williams TA, et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020 Oct;38(10):1929-36.
http://www.ncbi.nlm.nih.gov/pubmed/32890265?tool=bestpractice.com
CT may be misleading, as it cannot distinguish aldosterone-producing adenomas from non-functioning nodules.[6]Stowasser M, Gordon RD, Rutherford JC, et al. Diagnosis and management of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2001 Sep;2(3):156-69.
http://journals.sagepub.com/doi/pdf/10.3317/jraas.2001.022
http://www.ncbi.nlm.nih.gov/pubmed/11881117?tool=bestpractice.com
[27]Stowasser M, Gordon RD. Primary aldosteronism - careful investigation is essential and rewarding. Mol Cell Endocrinol. 2004 Mar 31;217(1-2):33-9.
http://www.ncbi.nlm.nih.gov/pubmed/15134798?tool=bestpractice.com
[71]Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511.
http://www.ncbi.nlm.nih.gov/pubmed/9221268?tool=bestpractice.com
[93]Doppman JL, Gill JR Jr, Miller DL, et al. Distinction between hyperaldosteronism due to bilateral hyperplasia and unilateral aldosteronoma: reliability of CT. Radiology. 1992 Sep;184(3):677-82.
http://www.ncbi.nlm.nih.gov/pubmed/1509049?tool=bestpractice.com
[94]Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004 Dec;136(6):1227-35.
http://www.ncbi.nlm.nih.gov/pubmed/15657580?tool=bestpractice.com
Similar limitations apply to adrenal MRI.[95]Tsushima Y, Ishizaka H, Matsumoto M. Adrenal masses: differentiation with chemical shift, fast low-angle shot MR imaging. Radiology. 1993 Mar;186(3):705-9.
http://www.ncbi.nlm.nih.gov/pubmed/8430178?tool=bestpractice.com
Responsiveness of plasma aldosterone (defined as a rise of at least 50% over basal) during 2 or 3 hours of upright posture following overnight recumbency or during angiotensin II infusion was once considered specific for BAH among patients with PA.[96]Ganguly AG, Melada GA, Luetscher JA, et al. Control of plasma aldosterone in primary aldosteronism: distinction between adenoma and hyperplasia. J Clin Endocrinol Metab. 1973 Nov;37(5):765-75.
http://www.ncbi.nlm.nih.gov/pubmed/4356136?tool=bestpractice.com
[97]Wisgerhof M, Brown RD, Hogan MJ, et al. The plasma aldosterone response to angiotensin II infusion in aldosterone-producing adenoma and idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 1981 Feb;52(2):195-8.
http://www.ncbi.nlm.nih.gov/pubmed/7462385?tool=bestpractice.com
However, similar findings are also observed in the angiotensin II-responsive variety of aldosterone-producing adenoma, which accounts for over 50% of aldosterone-producing adenomas in some series.[4]Gordon RD, Stowasser M, Klemm SA, et al. Primary aldosteronism - some genetic, morphological, and biochemical aspects of subtypes. Steroids. 1995 Jan;60(1):35-41.
http://www.ncbi.nlm.nih.gov/pubmed/7792813?tool=bestpractice.com
[98]Gordon RD, Hamlet SM, Tunny TJ, et al. Aldosterone-producing adenomas responsive to angiotensin pose problems in diagnosis. Clin Exp Pharmacol Physiol. 1987 Mar;14(3):175-9.
http://www.ncbi.nlm.nih.gov/pubmed/2822305?tool=bestpractice.com
[99]Gordon RD, Gomez-Sanchez CE, Hamlet SM, et al. Angiotensin-responsive aldosterone-producing adenoma masquerades as idiopathic hyperaldosteronism (IHA: adrenal hyperplasia) or low-renin essential hypertension. J Hypertens Suppl. 1987 Dec;5(5):S103-6.
http://www.ncbi.nlm.nih.gov/pubmed/2832571?tool=bestpractice.com
Examination of the aldosterone response to posture in patients with PA is nevertheless worthwhile, as its absence narrows the diagnosis to angiotensin II-unresponsive aldosterone-producing adenoma or FH-I in most cases. Hybrid steroid levels (18-hydroxy- and 18-oxo-cortisol) are elevated in FH-I and angiotensin II-unresponsive aldosterone-producing adenoma, and are useful evidence suggesting one or the other of these two conditions. However, they are not widely available, and because they are normal in both BAH and angiotensin II-responsive aldosterone-producing adenoma, they do not distinguish unilateral from bilateral PA.[91]Stowasser M, Bachmann AW, Jonsson JR, et al. Clinical, biochemical and genetic approaches to the detection of familial hyperaldosteronism type I. J Hypertens. 1995 Dec;13(12 Pt 2):1610-3.
http://www.ncbi.nlm.nih.gov/pubmed/8903619?tool=bestpractice.com
[98]Gordon RD, Hamlet SM, Tunny TJ, et al. Aldosterone-producing adenomas responsive to angiotensin pose problems in diagnosis. Clin Exp Pharmacol Physiol. 1987 Mar;14(3):175-9.
http://www.ncbi.nlm.nih.gov/pubmed/2822305?tool=bestpractice.com
[99]Gordon RD, Gomez-Sanchez CE, Hamlet SM, et al. Angiotensin-responsive aldosterone-producing adenoma masquerades as idiopathic hyperaldosteronism (IHA: adrenal hyperplasia) or low-renin essential hypertension. J Hypertens Suppl. 1987 Dec;5(5):S103-6.
http://www.ncbi.nlm.nih.gov/pubmed/2832571?tool=bestpractice.com
For the above reasons, adrenal venous sampling (AVS) is the only dependable way to differentiate bilateral from unilateral PA.[28]Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916.
https://academic.oup.com/jcem/article/101/5/1889/2804729
http://www.ncbi.nlm.nih.gov/pubmed/26934393?tool=bestpractice.com
[71]Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511.
http://www.ncbi.nlm.nih.gov/pubmed/9221268?tool=bestpractice.com
[94]Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004 Dec;136(6):1227-35.
http://www.ncbi.nlm.nih.gov/pubmed/15657580?tool=bestpractice.com
[100]Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics. 2005 Oct;25 Suppl 1:S143-58.
http://pubs.rsna.org/doi/full/10.1148/rg.25si055514
http://www.ncbi.nlm.nih.gov/pubmed/16227488?tool=bestpractice.com
[101]Yeung A, Friedmann P, In H, et al. Evaluation of adrenal vein sampling use and outcomes in patients with primary aldosteronism. J Surg Res. 2020 Dec;256:673-9.
http://www.ncbi.nlm.nih.gov/pubmed/32827833?tool=bestpractice.com
Some centres therefore recommend this procedure in all patients with PA (other than those with FH-I).[102]Sun F, Hong Y, Zhang H, et al. Determination of adrenal hypersecretion in primary aldosteronism without aldosterone-production adenomas. BMC Endocr Disord. 2021 May 31;21(1):114.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8167985
http://www.ncbi.nlm.nih.gov/pubmed/34059026?tool=bestpractice.com
Some guidelines suggest that AVS may be bypassed in patients aged <35 years with unilateral adenoma before proceeding to unilateral adrenalectomy.[10]Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021 Dec;9(12):876-92.
http://www.ncbi.nlm.nih.gov/pubmed/34798068?tool=bestpractice.com
[28]Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916.
https://academic.oup.com/jcem/article/101/5/1889/2804729
http://www.ncbi.nlm.nih.gov/pubmed/26934393?tool=bestpractice.com
[41]Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020 Oct;38(10):1919-28.
http://www.ncbi.nlm.nih.gov/pubmed/32890264?tool=bestpractice.com
[103]Yip L, Duh QY, Wachtel H, et al. American Association of Endocrine Surgeons guidelines for adrenalectomy: executive summary. JAMA Surg. 2022 Oct 1;157(10):870-7.
https://jamanetwork.com/journals/jamasurgery/fullarticle/2795363
http://www.ncbi.nlm.nih.gov/pubmed/35976622?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: CT showing lesion in right adrenal gland in patient with right aldosterone-producing adenomaFrom the personal collection of Dr Michael Stowasser; used with permission [Citation ends].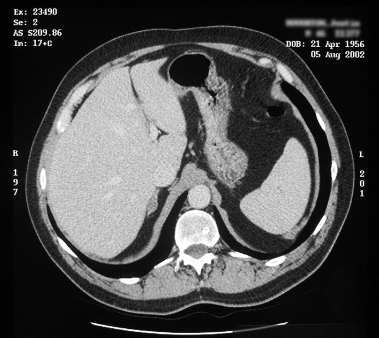 [Figure caption and citation for the preceding image starts]: Computed tomography (CT) showing lesion in right adrenal gland in patient with bilateral adrenal hyperplasiaFrom the personal collection of Dr Michael Stowasser; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Computed tomography (CT) showing lesion in right adrenal gland in patient with bilateral adrenal hyperplasiaFrom the personal collection of Dr Michael Stowasser; used with permission [Citation ends].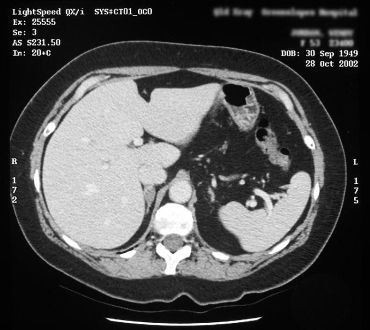
Nuclear imaging using positron emission tomography-computed tomography (PET-CT) with labelled metomidate as a ligand of CYP11B1 and CYP11B2 has been proposed to be a viable alternative to AVS, or as an adjunct to AVS in difficult cases.[104]Powlson AS, Gurnell M, Brown MJ. Nuclear imaging in the diagnosis of primary aldosteronism. Curr Opin Endocrinol Diabetes Obes. 2015 Jun;22(3):150-6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4405075
http://www.ncbi.nlm.nih.gov/pubmed/25871964?tool=bestpractice.com
[105]O'Shea PM, O'Donoghue D, Bashari W, et al. ¹¹ C-Metomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice. Clin Endocrinol (Oxf). 2019 May;90(5):670-9.
http://www.ncbi.nlm.nih.gov/pubmed/30721535?tool=bestpractice.com
In this protocol dexamethasone pre-treatment is used to suppress adrenocorticotropic hormone and CYP11B1 expression. The short half-life and lack of specificity for CYP11B2 of the currently used isotope limits the widespread application and reliability of this technique, however, work is ongoing to find alternatives.
 [Figure caption and citation for the preceding image starts]: Computed tomography (CT) showing lesion in right adrenal gland in patient with bilateral adrenal hyperplasiaFrom the personal collection of Dr Michael Stowasser; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Computed tomography (CT) showing lesion in right adrenal gland in patient with bilateral adrenal hyperplasiaFrom the personal collection of Dr Michael Stowasser; used with permission [Citation ends].