Approach
Diagnosis is based on clinical signs and symptoms. There are no unique laboratory diagnostic tests for the disease. The principal signs were recognised and reported in 1974, and these criteria have been updated by the American Heart Association and endorsed by the American Academy of Pediatrics.[1][29]
The Single Hub and Access point for paediatric Rheumatology in Europe (SHARE) guidelines recommend consideration of the diagnosis of Kawasaki disease (KD) in any child with a febrile, exanthematous illness, with systemic inflammation, especially if fever persists for longer than 4 days.[30]
Acute stage
The acute stage generally lasts 7-11 days.
In addition to fever, patients must have four more of the following five signs and symptoms:
Polymorphous erythematous rash
Non-purulent bilateral conjunctival injection (occurs in 90%)
Oropharyngeal changes, including diffuse hyperaemia, strawberry tongue, and lip changes (e.g., swelling, fissuring, erythema, and bleeding)
Peripheral extremity changes, including erythema, oedema, induration, and desquamation, which may cause difficulty walking
Non-purulent cervical lymphadenopathy. This occurs in 40% of cases (although other reports are 50% to 75%) and is generally a single, enlarged, non-suppurative cervical node measuring approximately ≥1.5 cm.
These criteria are only guidelines in order to prevent misdiagnosis or over-diagnosis. According to these guidelines, a diagnosis can be made on day 4 of the fever if four principal criteria are met, especially when redness and swelling of the hands and feet are present. Experienced clinicians who have treated many KD patients, in rare instances, may establish diagnosis on day 3 of the fever in the presence of a classic clinical presentation.[1] The fever must be high; usually greater than 39°C (102°F), but is often over 39.9°C (104°F). Patients are often irritable beyond that expected for the extent of fever.
The European SHARE recommends that a diagnosis can be reached before 5 days of fever if four principal criteria are met or if there is evidence of coronary artery dilation (Z score >2 but <2.5) or aneurysm (Z score ≥2.5) or if there is evidence of persistent inflammation, with no alternative diagnosis, and a clinical suspicion of KD.[31]
The diagnosis of incomplete KD can be made according to the American Heart Association (AHA) 2017 guidelines in a child with 5 or more days of fever and two or three clinical criteria or in infants with ≥7 days of fever without alternative diagnosis.[1] The SHARE guidelines highlight that this is a pragmatic approach to this situation but lacks an evidence base and studies validating criteria for incomplete KD is an important research priority.[31] Echocardiography in this scenario showing coronary vasculitis would confirm the diagnosis, but a normal echocardiogram does not exclude the diagnosis. Moreover, incomplete KD occurs most commonly in infants who are at risk of developing coronary artery abnormalities and who may have prolonged fever as the only clinical finding. In patients with suspected KD, the presence of irritability or erythema and/or induration at the site of previous BCG vaccination are signs that increase the likelihood of diagnosis.[31][Figure caption and citation for the preceding image starts]: SHARE recommendations for the management of suspected incomplete Kawasaki diseasede Graeff N, Groot N, Ozen S, et al. European consensus-based recommendations for the diagnosis and treatment of Kawasaki disease - the SHARE initiative. Rheumatology (Oxford). 2019;58(4):672-682. [Citation ends].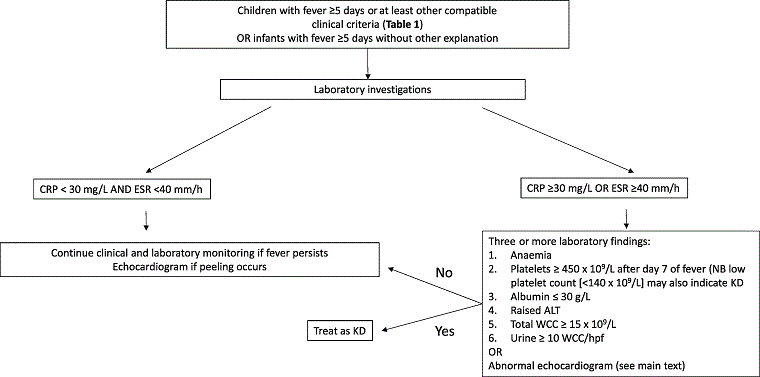
Although not part of the formal diagnostic criteria, the 2017 AHA guidelines emphasise the use of coronary artery measurement Z scores, which reflect standardised dimensions normalised for body surface area (BSA) and allow for classification and comparisons across time and populations.[1][32]
Echocardiography is considered positive for coronary abnormalities if any of the following conditions are met:[1]
Left anterior descending coronary artery or right coronary artery Z score ≥2.5
Coronary artery aneurysm seen
Three or more other suggestive features, including decreased left ventricular function, mitral regurgitation, pericardial effusion, or Z scores in left anterior descending coronary artery or right coronary artery of 2.0 to 2.5.
In addition, the presence of three or more of the following laboratory features may increase the index of suspicion for KD: 1) anaemia; 2) platelet count of >450,000 after day 7 of fever; 3) albumin <3.0 g/dL; 4) elevated alanine aminotransferase (ALT); 5) white blood cell (WBC) count >15,000; 6) urine with >10 WBC/high power field.[1]
In the absence of a diagnostic test, these criteria become pivotal in diagnosing a patient with KD. Some laboratory tests may be supportive, such as acute phase reactants, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). These are significantly raised (to a greater degree than expected for common viral infections).[33]
Uncommon findings on physical examination in acute stage
Less common findings may include: stiff neck secondary to aseptic meningitis, facial palsy, anterior uveitis (70%), pleural effusion, pulmonary infiltrates, pericardial effusion with or without myocarditis, and congestive heart failure.
Others findings include abdominal pain, vomiting, pseudo-obstruction, diarrhoea, hepatitis, obstructive jaundice, gallbladder distension or hydrops of the gallbladder, pancreatitis, joint involvement (arthralgias or arthritis), meatitis, vulvitis, urethritis with sterile pyuria, proteinuria, nephritis, and acute renal failure. In addition, peripheral extremity gangrene, pustules, erythema multiforme-like lesions, perineal erythema (50% to 70%), macules, papules, measles-like rash, and scarlet fever-like erythema may be found.
Subacute stage
This stage lasts between 2-3 weeks, during which the presenting signs and symptoms are in the process of resolving, including among others, the persistent irritability and reduced appetite. Conjunctival injection and cracking of the lips may persist in this phase.
Typically features during this phase of illness include peripheral desquamation, improving acute phase biomarkers, development of thrombocytosis, coronary artery aneurysm development and reduced temperature.
Convalescent/chronic stage
This stage lasts between 4-6 weeks; the key signs and symptoms have resolved and inflammatory markers normalised. However, in patients who have developed cardiac sequelae, coronary artery aneurysms can persist and worsen, and life-threatening complications can develop including thrombosis or myocardial infarction. 60% of small aneurysms will resolve.
Risk stratification in the long term
In the longer term, risk stratification is based primarily on maximal coronary artery luminal dimensions, normalised as Z scores, and takes into account both past and current coronary involvement.
No involvement: always <2
Dilation only: 2.0 to <2.5; or if initially <2, a decrease in Z score during follow-up ≥1
Small aneurysm: ≥2.5 to <5.0
Medium aneurysm: ≥5 to <10, and absolute dimension <8 mm
Large or giant aneurysm: ≥10, or absolute dimension ≥8 mm.
Additional clinical features indicating risk of myocardial ischaemia include greater total number, length, number of branches, and distal location of aneurysms; structural and functional vessel wall abnormalities; poor collateral vessels; previous revascularisation, coronary artery thrombosis, or myocardial infarction; and ventricular dysfunction.[1]
Initial investigations
The European SHARE guidelines recommend the following lab tests that should be monitored in all patients: erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), full blood count, liver function, serum albumin, serum sodium (for hyoponatraemia associated with severe disease), renal function and urinalysis. Ferritin and fibrinogen should also be monitored if macrophage activation syndrome is suspected. Notably, ESR is not a particularly useful test after intravenous immunoglobulin treatment as it may become elevated due to binding of immunoglobin to red blood cells.[1][31]
During the acute stage, many acute-phase reactant markers such as ESR, CRP, and serum ferritin are significantly raised. These tests tend to return to normal levels at the end of the subacute phase towards the convalescent phase, with CRP returning to normal values more rapidly than ESR. If ESR and CRP were normal or very mildly raised (ESR <40 mm/hour and/or CRP <190 nm/L [<20 mg/L or<2 mg/dL]) at the onset of the acute stage, this would be atypical of KD and alternative diagnoses should be considered including streptococcal infections (especially scarlet fever), and viral illnesses, with infectious disease consultation if necessary. Mild to moderate normochromic anaemia is observed in the acute stage, along with an elevated level of white blood cell count. During the subacute stage, thrombocytosis is typical with platelet count increasing during the second week and continuing for the third week, usually with platelet counts of up to 1000 x 10⁹/L (1 million/microlitre), but counts as high as 2000 x 10⁹/L (2 million/microlitre) are occasionally observed.
The European SHARE guidelines recommend an ECG and echocardiogram at 6-8 weeks after disease onset and at least weekly in patients with confirmed coronary abnormalities or ongoing inflammation.[31] The 2017 AHA guidelines recommend that patients with coronary artery abnormalities (Z score >2.5) during the acute illness should have more frequent echocardiography (at least twice weekly) until dimensions cease, progressing to monitor risk of and presence of thrombus.[1] The SHARE guidelines recommend that patients with a normal initial echocardiogram in whom the acute illness has subsided should have a repeat echocardiogram at 2 weeks after initiation of treatment.[31]The 2017 AHA guidelines similarly recommends an echocardiogram at 1-2 weeks and 4-6 weeks for these patients in whom the initial echocardiogram was normal.[1]
These recommendations have been made to assess for possible expansion of aneurysms due to the associated thrombosis risk and consequent morbidity and mortality risk. Perform echocardiography for patients with expanding large or giant aneurysms twice per week while dimensions are expanding rapidly, at least once weekly in the first 45 days of illness, and then monthly until the third month after illness onset.[1]
Be aware that in the first week of illness, the echocardiogram is typically normal and does not rule out the diagnosis.[1] If the echocardiographic findings are abnormal at any stage in the course of the illness, refer the patient to a paediatric cardiologist for a complete cardiac work-up and follow-up care.
Other investigations
Additional tests are performed to exclude or identify other organ system involvement:
Liver function tests: should be done routinely in all patients with suspected KD to assess for hepatitis. Transaminitis is a common finding in KD. The patient may have abdominal pain, jaundice, and nausea and/or vomiting in addition to high fever.
Urinalysis: should be done routinely in all patients with suspected KD; it will show a mild to moderate sterile pyuria of urethral origin in 50% of patients. If urinalysis is abnormal, a culture should be performed to rule out a urinary tract infection.
Chest x-ray: performed if pericarditis or pneumonitis suspected.
Electrocardiogram: to exclude conduction abnormalities.
Ultrasonography of the gallbladder: to exclude hydrops of the gallbladder (if suspected).
Ultrasonography of the testes: to exclude epididymitis (if suspected).
Lumbar puncture: performed if patients present with nuchal rigidity and high fever. This test is necessary to exclude meningitis.
Emerging tests
Magnetic resonance angiography and cardiac catheterisation with angiography may be recommended to further assess coronary aneurysms, and are superior to echocardiography, although may be challenging to perform in young children <15 kg in weight, where computed tomographic angiography may be preferred. These imaging modalities are usually recommended by cardiology, especially in the context of unclear findings on echocardiography or in the presence of giant aneurysms. These tests are the responsibility of the cardiologist and would be ordered when the echocardiogram findings are not clear or when the echocardiogram shows giant aneurysms and need for more detailed anatomical delineation. Circulating N-terminal pro-brain natriuretic peptide (NT-proBNP) has been proposed as a potential diagnostic biomarker for KD. One meta-analysis reviewing the diagnostic utility of NT-proBNP showed that although it has moderate efficacy for diagnosing KD, in isolation, it was not helpful in confirming or refuting the diagnosis.[34]
Use of this content is subject to our disclaimer