Chronic rhinosinusitis without nasal polyps
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
chronic rhinosinusitis
nasal saline irrigation
Initial treatment recommended by American and European guidelines includes nasal saline irrigation.[3]Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021 Mar;11(3):213-739. https://www.doi.org/10.1002/alr.22741 http://www.ncbi.nlm.nih.gov/pubmed/33236525?tool=bestpractice.com [20]Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(suppl 2):S1-S39. http://oto.sagepub.com/content/152/2_suppl/S1.long http://www.ncbi.nlm.nih.gov/pubmed/25832968?tool=bestpractice.com [21]Peters AT, Spector S, Hsu J, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014 Oct;113(4):347-85. http://www.ncbi.nlm.nih.gov/pubmed/25256029?tool=bestpractice.com [27]Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020 Feb 20;58(suppl s29):1-464. http://www.ncbi.nlm.nih.gov/pubmed/32077450?tool=bestpractice.com [28]Mösges R, Heubach CP. What is the evidence for non-antibiotic drug therapy of rhinosinusitis? Laryngorhinootologie. 2011 Dec;90(12):740-6. http://www.ncbi.nlm.nih.gov/pubmed/22016266?tool=bestpractice.com [29]Chong LY, Head K, Hopkins C, et al. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011996. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011996.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27115217?tool=bestpractice.com [30]Chong LY, Head K, Hopkins C, et al. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011993. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011993.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27115215?tool=bestpractice.com [31]Wang C, Lou H, Wang X, et al. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2015 Apr;135(4):922-29. http://www.jacionline.org/article/S0091-6749(14)01513-9/fulltext http://www.ncbi.nlm.nih.gov/pubmed/25483598?tool=bestpractice.com [32]Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015 Sep 1;314(9):926-39. http://www.ncbi.nlm.nih.gov/pubmed/26325561?tool=bestpractice.com [33]Chong LY, Head K, Hopkins C, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011995. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011995.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27115216?tool=bestpractice.com
Nasal saline irrigations should be used throughout the treatment for both acute and chronic rhinosinusitis. Irrigating debris as well as inflammatory molecules improves secretory stasis and helps to improve nasal congestion and obstruction. Easily performed and usually well tolerated, irrigations have been shown to be safe and beneficial.[28]Mösges R, Heubach CP. What is the evidence for non-antibiotic drug therapy of rhinosinusitis? Laryngorhinootologie. 2011 Dec;90(12):740-6. http://www.ncbi.nlm.nih.gov/pubmed/22016266?tool=bestpractice.com [32]Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015 Sep 1;314(9):926-39. http://www.ncbi.nlm.nih.gov/pubmed/26325561?tool=bestpractice.com [33]Chong LY, Head K, Hopkins C, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011995. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011995.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27115216?tool=bestpractice.com A duration of treatment of >8 weeks is recommended.[3]Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021 Mar;11(3):213-739. https://www.doi.org/10.1002/alr.22741 http://www.ncbi.nlm.nih.gov/pubmed/33236525?tool=bestpractice.com
Saline irrigation can be done with a high-volume, high-flow device (e.g., squeeze bottle) two to three times daily to aid in clearance of mucus. Devices with volume of >60 mL have greater benefits.[3]Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021 Mar;11(3):213-739. https://www.doi.org/10.1002/alr.22741 http://www.ncbi.nlm.nih.gov/pubmed/33236525?tool=bestpractice.com
intranasal corticosteroid
Treatment recommended for ALL patients in selected patient group
Initial treatment recommended by American and European guidelines includes topical intranasal corticosteroids.[3]Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021 Mar;11(3):213-739. https://www.doi.org/10.1002/alr.22741 http://www.ncbi.nlm.nih.gov/pubmed/33236525?tool=bestpractice.com [20]Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(suppl 2):S1-S39. http://oto.sagepub.com/content/152/2_suppl/S1.long http://www.ncbi.nlm.nih.gov/pubmed/25832968?tool=bestpractice.com [21]Peters AT, Spector S, Hsu J, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014 Oct;113(4):347-85. http://www.ncbi.nlm.nih.gov/pubmed/25256029?tool=bestpractice.com [27]Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020 Feb 20;58(suppl s29):1-464. http://www.ncbi.nlm.nih.gov/pubmed/32077450?tool=bestpractice.com [28]Mösges R, Heubach CP. What is the evidence for non-antibiotic drug therapy of rhinosinusitis? Laryngorhinootologie. 2011 Dec;90(12):740-6. http://www.ncbi.nlm.nih.gov/pubmed/22016266?tool=bestpractice.com [29]Chong LY, Head K, Hopkins C, et al. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011996. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011996.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27115217?tool=bestpractice.com [30]Chong LY, Head K, Hopkins C, et al. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011993. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011993.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27115215?tool=bestpractice.com [31]Wang C, Lou H, Wang X, et al. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2015 Apr;135(4):922-29. http://www.jacionline.org/article/S0091-6749(14)01513-9/fulltext http://www.ncbi.nlm.nih.gov/pubmed/25483598?tool=bestpractice.com [32]Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015 Sep 1;314(9):926-39. http://www.ncbi.nlm.nih.gov/pubmed/26325561?tool=bestpractice.com [33]Chong LY, Head K, Hopkins C, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011995. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011995.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27115216?tool=bestpractice.com Corticosteroid nasal irrigations are recommended in postoperative patients and as an option for those undergoing non-surgical therapy.[3]Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021 Mar;11(3):213-739. https://www.doi.org/10.1002/alr.22741 http://www.ncbi.nlm.nih.gov/pubmed/33236525?tool=bestpractice.com
Can be used long term. Safe and effective in chronic rhinosinusitis with and without polyps.[28]Mösges R, Heubach CP. What is the evidence for non-antibiotic drug therapy of rhinosinusitis? Laryngorhinootologie. 2011 Dec;90(12):740-6.
http://www.ncbi.nlm.nih.gov/pubmed/22016266?tool=bestpractice.com
[29]Chong LY, Head K, Hopkins C, et al. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011996.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011996.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27115217?tool=bestpractice.com
[30]Chong LY, Head K, Hopkins C, et al. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011993.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011993.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27115215?tool=bestpractice.com
[31]Wang C, Lou H, Wang X, et al. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2015 Apr;135(4):922-29.
http://www.jacionline.org/article/S0091-6749(14)01513-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25483598?tool=bestpractice.com
[32]Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015 Sep 1;314(9):926-39.
http://www.ncbi.nlm.nih.gov/pubmed/26325561?tool=bestpractice.com
[  ]
In people with chronic rhinosinusitis, how do intranasal steroids compare with placebo?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1366/fullShow me the answer
[
]
In people with chronic rhinosinusitis, how do intranasal steroids compare with placebo?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1366/fullShow me the answer
[  ]
In people with chronic rhinosinusitis, how do high-dose intranasal corticosteroids compare with low-dose at improving outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1470/fullShow me the answer
]
In people with chronic rhinosinusitis, how do high-dose intranasal corticosteroids compare with low-dose at improving outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1470/fullShow me the answer
Mucosal shrinkage allows for sinus drainage with decrease in overall mucus production.
Risks are very small. Epistaxis is a common complaint.[29]Chong LY, Head K, Hopkins C, et al. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011996.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011996.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27115217?tool=bestpractice.com
[30]Chong LY, Head K, Hopkins C, et al. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011993.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011993.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27115215?tool=bestpractice.com
[  ]
In people with chronic rhinosinusitis, how do intranasal steroids compare with placebo?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1366/fullShow me the answer
[
]
In people with chronic rhinosinusitis, how do intranasal steroids compare with placebo?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1366/fullShow me the answer
[  ]
In people with chronic rhinosinusitis, how do high-dose intranasal corticosteroids compare with low-dose at improving outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1470/fullShow me the answer Patients who have not been instructed in proper use will often spray directly onto the septum. This causes excoriation of the Kiesselbach area (anterior septum) and subsequent nosebleeds. Key to avoidance of bleeds is proper use by directing the spray laterally towards the outside wall of the nose away from the midline septum. Routine nasal hygiene and humidification reduce this risk further.
]
In people with chronic rhinosinusitis, how do high-dose intranasal corticosteroids compare with low-dose at improving outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1470/fullShow me the answer Patients who have not been instructed in proper use will often spray directly onto the septum. This causes excoriation of the Kiesselbach area (anterior septum) and subsequent nosebleeds. Key to avoidance of bleeds is proper use by directing the spray laterally towards the outside wall of the nose away from the midline septum. Routine nasal hygiene and humidification reduce this risk further.
Systemic absorption is negligible. Rare fungal superinfections occur.
Primary options
budesonide nasal: 32-64 micrograms (1-2 sprays) in each nostril once daily, maximum 256 micrograms/day
OR
fluticasone propionate nasal: 100 micrograms (2 sprays) in each nostril once daily; or 50 micrograms (1 spray) in each nostril twice daily
OR
mometasone nasal: 100 micrograms (2 sprays) in each nostril once daily
OR
triamcinolone nasal: 110 micrograms (2 sprays) in each nostril once daily
OR
flunisolide nasal: 50 micrograms (2 sprays) in each nostril twice daily
antibiotic therapy
Additional treatment recommended for SOME patients in selected patient group
Oral antibiotics may be considered depending on examination/endoscopic findings, and response to initial treatment. If used, antibiotics should ideally be based on cultures.[21]Peters AT, Spector S, Hsu J, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014 Oct;113(4):347-85. http://www.ncbi.nlm.nih.gov/pubmed/25256029?tool=bestpractice.com
No antibiotics are US Food and Drug Administration (FDA)-approved for use in chronic rhinosinusitis. The rationale for treatment in chronic rhinosinusitis is to eradicate bacterial infection. Antibiotics are also used in the short-term for acute exacerbations, with a treatment duration shorter than 4 weeks.[21]Peters AT, Spector S, Hsu J, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014 Oct;113(4):347-85. http://www.ncbi.nlm.nih.gov/pubmed/25256029?tool=bestpractice.com Longer term antibiotics (≥4 weeks) may be considered if nasal saline irrigation and topical intranasal corticosteroids have not led to an improvement in symptoms after 3 months, particularly if the patient’s main complaint is discharge/facial pain.[27]Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020 Feb 20;58(suppl s29):1-464. http://www.ncbi.nlm.nih.gov/pubmed/32077450?tool=bestpractice.com
Prolonged antibiotics may be utilised in patients with persistent purulence, but the evidence is not very strong and this is not common in practice.[21]Peters AT, Spector S, Hsu J, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014 Oct;113(4):347-85. http://www.ncbi.nlm.nih.gov/pubmed/25256029?tool=bestpractice.com One systematic review recommended a short (<3 weeks) course of antibiotics as a treatment option, except for macrolides, for which there is evidence of effectiveness for longer courses in selected patients.[34]Soler ZM, Oyer SL, Kern RC, et al. Antimicrobials and chronic rhinosinusitis with or without polyposis in adults: an evidenced-based review with recommendations. Int Forum Allergy Rhinol. 2013 Jan;3(1):31-47. http://www.ncbi.nlm.nih.gov/pubmed/22736403?tool=bestpractice.com One Cochrane review found moderate-quality evidence of a modest improvement in disease‐specific quality of life in adults with chronic rhinosinusitis, without polyps, who had received three months of a macrolide antibiotic.[35]Head K, Chong LY, Piromchai P, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016 Apr 26;(4):CD011994. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011994.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/27113482?tool=bestpractice.com
Risks include allergic reaction and development of antibiotic resistance. Regional drug susceptibility patterns should be considered.
Culture-directed antibiotic therapy (based on endoscopically obtained samples from the middle meatus) is recommended for patients with infection refractory to initial antibiotic treatment.
The FDA has issued a safety warning stating that serious side effects associated with fluoroquinolones outweigh the benefits for patients with rhinosinusitis, bronchitis, and uncomplicated urinary tract infections who have other treatment options.[36]US Food and Drug Administration. Fluoroquinolone antibacterial drugs: drug safety communication - FDA advises restricting use for certain uncomplicated infections. May 2016 [internet publication]. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf The FDA advises that for patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options.
Primary options
amoxicillin/clavulanate: chronic: 250 mg orally three times daily for 3-4 weeks; or 500 mg orally twice daily for 3-4 weeks; acute exacerbations: 500 mg three times daily for 10 days; or 875 mg twice daily for 10 days
More amoxicillin/clavulanateDose refers to amoxicillin component.
OR
cefuroxime: 250-500 mg orally twice daily for at least 10 days
OR
doxycycline: 200 mg orally on the first day, followed by 100 mg once daily for 20 days
Secondary options
clarithromycin: 250-500 mg orally (immediate-release) twice daily for 10 days or preferably 3-4 weeks
OR
clindamycin: 300 mg orally four times daily for at least 10 days
More clindamycinReserved for resistant Staphylococcus aureus.
OR
levofloxacin: chronic: 500 mg orally once daily for 3-4 weeks; acute exacerbations: 750 mg orally once daily for 5 days
More levofloxacinReserved for those who do not have alternative treatment options.
decongestant
Additional treatment recommended for SOME patients in selected patient group
Decongestants can be used for symptomatic relief. They help to reduce tissue oedema, facilitate drainage, and promote patency of sinus ostia.
Available in topical and oral forms, each differing slightly in its method of action. Topical agents provide almost immediate symptomatic relief by shrinking inflamed and swollen nasal mucosa. Topical nasal formulations should not be used for longer than 3-5 consecutive days because of the risk of tolerance, rhinitis medicamentosa, and rebound after drug withdrawal.
Oral systemic agents are used when decongestion is necessary for >3 days. They are alpha-adrenergic agonists that reduce nasal blood flow.
The sympathomimetic decongestant pseudoephedrine can be considered a secondary option. However, pseudoephedrine-containing medications are associated with a risk of posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome. These are rare conditions with potentially serious and life-threatening complications.
Pseudoephedrine-containing medications should not be used in patients with severe or uncontrolled hypertension, or those with severe acute or chronic renal disease or failure.[46]European Medicines Agency. Pseudoephedrine-containing medicinal products - referral. Mar 2024 [internet publication]. https://www.ema.europa.eu/en/medicines/human/referrals/pseudoephedrine-containing-medicinal-products
Primary options
oxymetazoline nasal: (0.05%) 2-3 sprays in each nostril twice daily, maximum 6 sprays/day
OR
phenylephrine nasal: (0.25 to 1%) 1-2 drops/sprays in each nostril every 4 hours when required
Secondary options
pseudoephedrine: 60 mg orally (immediate-release) every 6 hours when required; or 120 mg orally (extended-release) every 12 hours when required; maximum 240 mg/day
antihistamine or leukotriene receptor antagonist
Additional treatment recommended for SOME patients in selected patient group
Antihistamines can be used selectively if there is an apparent allergic component to sinus symptoms (e.g., sneezing, itchy eyes, and rhinorrhoea), which are often seasonal. Empirical treatment with oral antihistamine can be attempted initially. Some patients prefer direct treatment via topical intranasal antihistamine. Patients who do not improve may be candidates for allergy testing.
Leukotriene receptor antagonists can be used as second line. They inhibit the leukotriene pathway involved with inflammation. Initially developed for patients with asthma, they have been advocated for patients with asthma, allergic rhinitis, and/or chronic rhinosinusitis.[32]Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015 Sep 1;314(9):926-39. http://www.ncbi.nlm.nih.gov/pubmed/26325561?tool=bestpractice.com [37]Steinke JW, Borish L. Leukotriene receptors in rhinitis and sinusitis. Curr Allergy Asthma Rep. 2004 May;4(3):217-23. http://www.ncbi.nlm.nih.gov/pubmed/15056404?tool=bestpractice.com
The US Food and Drug Administration (FDA) has strengthened its warning for montelukast (a leukotriene receptor antagonist) about serious behaviour- and mood-related changes. For allergic rhinitis, the FDA has determined that montelukast should be reserved for those who are not treated effectively with, or cannot tolerate, other allergy medicines.[38]US Food & Drug Administration. FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. 4 March 2020 [internet publication]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug
Primary options
loratadine: 10 mg orally once daily when required
OR
fexofenadine: 60 mg orally every 12 hours when required; or 180 mg orally once daily when required
OR
desloratadine: 5 mg orally once daily when required
OR
chlorphenamine: 4 mg orally (immediate-release) every 4-6 hours when required, maximum 24 mg/day
OR
azelastine nasal: 2 sprays in each nostril every 12 hours when required
OR
cetirizine: 5-10 mg/day orally given as a single dose or in 2 divided doses when required
Secondary options
zafirlukast: 20 mg orally twice daily
OR
montelukast: 10 mg orally once daily
OR
zileuton: 1200 mg orally (extended-release) twice daily
continued symptoms despite medical therapy
endoscopic sinus surgery
Significantly symptomatic patients failing medical therapy, with sinus inflammation on post-therapy computed tomography (CT) are considered surgical candidates. For patients with extensive disease, previous surgery, or need for surgery in anatomically sensitive areas (e.g., frontal or sphenoid sinuses), image-guided surgery is advocated. The CT scan is downloaded onto a computer and the patient's head is matched to CT scan coordinates pre-operatively.[Figure caption and citation for the preceding image starts]: Pre-op planning at a surgical navigation workstationFrom the personal collection of Dr Raj Sindwani; used with permission [Citation ends].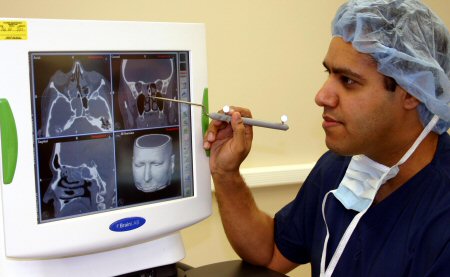 The system tracks the tips of surgical instruments in the sinuses and the patient's head using infrared/electromagnetic technology.[Figure caption and citation for the preceding image starts]: Image-guided endoscopic sinus surgery using an optical-based surgical navigation systemFrom the personal collection of Dr Raj Sindwani; used with permission [Citation ends].
The system tracks the tips of surgical instruments in the sinuses and the patient's head using infrared/electromagnetic technology.[Figure caption and citation for the preceding image starts]: Image-guided endoscopic sinus surgery using an optical-based surgical navigation systemFrom the personal collection of Dr Raj Sindwani; used with permission [Citation ends]. Complications of sinus surgery include bleeding, infection, brain injury/cerebrospinal fluid leak, orbital injury (bruising, double vision/blindness), residual/recurrent sinus disease, and the need for revision surgery. Major complications (intracranial and orbital) are very rare (<1%).[37]Steinke JW, Borish L. Leukotriene receptors in rhinitis and sinusitis. Curr Allergy Asthma Rep. 2004 May;4(3):217-23.
http://www.ncbi.nlm.nih.gov/pubmed/15056404?tool=bestpractice.com
[41]Mehta U, Huber TC, Sindwani R. Patient expectations and recovery following endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2006 Mar;134(3):483-7.
http://www.ncbi.nlm.nih.gov/pubmed/16500449?tool=bestpractice.com
When counselling patients before surgery, it is important to note that nasal obstruction improves the most, facial pain and discharge improve moderately, while headache and hyposmia improve least.[43]Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009 May;140(5):633-9.
http://www.ncbi.nlm.nih.gov/pubmed/19393402?tool=bestpractice.com
Fatigue and body pain also improve after endoscopic sinus surgery.[44]Chester AC, Sindwani R, Smith TL, et al. Systematic review of change in bodily pain after sinus surgery. Otolaryngol Head Neck Surg. 2008 Dec;139(6):759-65.
http://www.ncbi.nlm.nih.gov/pubmed/19041499?tool=bestpractice.com
[45]Chester AC, Sindwani R, Smith TL, et al. Fatigue improvement following endoscopic sinus surgery: a systematic review and meta-analysis. Laryngoscope. 2008 Apr;118(4):730-9.
http://www.ncbi.nlm.nih.gov/pubmed/18216743?tool=bestpractice.com
Complications of sinus surgery include bleeding, infection, brain injury/cerebrospinal fluid leak, orbital injury (bruising, double vision/blindness), residual/recurrent sinus disease, and the need for revision surgery. Major complications (intracranial and orbital) are very rare (<1%).[37]Steinke JW, Borish L. Leukotriene receptors in rhinitis and sinusitis. Curr Allergy Asthma Rep. 2004 May;4(3):217-23.
http://www.ncbi.nlm.nih.gov/pubmed/15056404?tool=bestpractice.com
[41]Mehta U, Huber TC, Sindwani R. Patient expectations and recovery following endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2006 Mar;134(3):483-7.
http://www.ncbi.nlm.nih.gov/pubmed/16500449?tool=bestpractice.com
When counselling patients before surgery, it is important to note that nasal obstruction improves the most, facial pain and discharge improve moderately, while headache and hyposmia improve least.[43]Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: a systematic review. Otolaryngol Head Neck Surg. 2009 May;140(5):633-9.
http://www.ncbi.nlm.nih.gov/pubmed/19393402?tool=bestpractice.com
Fatigue and body pain also improve after endoscopic sinus surgery.[44]Chester AC, Sindwani R, Smith TL, et al. Systematic review of change in bodily pain after sinus surgery. Otolaryngol Head Neck Surg. 2008 Dec;139(6):759-65.
http://www.ncbi.nlm.nih.gov/pubmed/19041499?tool=bestpractice.com
[45]Chester AC, Sindwani R, Smith TL, et al. Fatigue improvement following endoscopic sinus surgery: a systematic review and meta-analysis. Laryngoscope. 2008 Apr;118(4):730-9.
http://www.ncbi.nlm.nih.gov/pubmed/18216743?tool=bestpractice.com
oral corticosteroid
Additional treatment recommended for SOME patients in selected patient group
Used in preparation for surgery and in acute exacerbations.[27]Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020 Feb 20;58(suppl s29):1-464. http://www.ncbi.nlm.nih.gov/pubmed/32077450?tool=bestpractice.com
Primary options
methylprednisolone: 24 mg orally on day one, decrease by 4 mg/day increments each day for 5 days

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer