Although MERS has not yet reached pandemic potential, it is a potentially severe infection with a high case fatality rate. Therefore, quick diagnosis is essential to prevent transmission and to provide supportive care in a timely manner. Physicians should have a high index of suspicion for all patients who present with fever and/or respiratory symptoms in the correct epidemiological context (i.e., travel from the Middle East), and these patients should be promptly evaluated. There are no pathognomonic features; therefore, molecular and serological testing is required to confirm the diagnosis. Co-infection with other respiratory viruses has been reported.
MERS is a notifiable disease and all suspected and confirmed cases should be reported to the appropriate authority.
Infection prevention and control measures
Isolation procedures should be initiated in all suspected or confirmed cases of MERS. An increased level of infection control precautions is recommended. Standard, droplet, and contact precautions are recommended, as well as airborne precautions, particularly when performing aerosol-generating procedures.[68]World Health Organization. Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Interim guidance. Oct 2019 [internet publication].
https://apps.who.int/iris/handle/10665/174652
[69]Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS): prevention and control for hospitalized MERS patients. May 2024 [internet publication].
https://www.cdc.gov/mers/hcp/infection-control
Detailed infection prevention and control recommendations are available from the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).[68]World Health Organization. Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Interim guidance. Oct 2019 [internet publication].
https://apps.who.int/iris/handle/10665/174652
[69]Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS): prevention and control for hospitalized MERS patients. May 2024 [internet publication].
https://www.cdc.gov/mers/hcp/infection-control
Diagnostic laboratory work on clinical specimens from patients who are suspected or confirmed to be infected should be conducted under Biosafety Level (BSL-2) practices and procedures.
History
A detailed history helps to clarify the level of risk for MERS and assess the possibility of other causes. Obtaining an epidemiological history is crucial for timely diagnosis and preventing potential outbreaks.[19]Lee SI. Costly lessons from the 2015 Middle East respiratory syndrome coronavirus outbreak in Korea. J Prev Med Public Health. 2015 Nov;48(6):274-6.
https://www.jpmph.org/journal/view.php?doi=10.3961/jpmph.15.064
http://www.ncbi.nlm.nih.gov/pubmed/26639740?tool=bestpractice.com
[21]Ha KM. A lesson learned from the MERS outbreak in South Korea in 2015. J Hosp Infect. 2016 Mar;92(3):232-4.
https://www.journalofhospitalinfection.com/article/S0195-6701(15)00397-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26601605?tool=bestpractice.com
All confirmed cases have either travelled to, resided in, or been in contact with someone who has travelled to the Middle East in the 14 days prior to the onset of symptoms.[17]Guery B, Poissy J, el Mansouf L, et al. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013 Jun 29;381(9885):2265-72.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)60982-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/23727167?tool=bestpractice.com
[19]Lee SI. Costly lessons from the 2015 Middle East respiratory syndrome coronavirus outbreak in Korea. J Prev Med Public Health. 2015 Nov;48(6):274-6.
https://www.jpmph.org/journal/view.php?doi=10.3961/jpmph.15.064
http://www.ncbi.nlm.nih.gov/pubmed/26639740?tool=bestpractice.com
[64]Reuss A, Litterst A, Drosten C, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20:620-5.
https://wwwnc.cdc.gov/eid/article/20/4/13-1375_article
http://www.ncbi.nlm.nih.gov/pubmed/24655721?tool=bestpractice.com
[65]Bialek SR, Allen D, Alvarado-Ramy F, et al. First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities - May 2014. MMWR Morb Mortal Wkly Rep. 2014;63:431-6.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6319a4.htm
http://www.ncbi.nlm.nih.gov/pubmed/24827411?tool=bestpractice.com
This includes the Arabian Peninsula (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates, Yemen) and its surrounding countries (Iraq; Iran; Israel, the West Bank, and Gaza; Jordan; Lebanon; Syria). Failure to recognise this risk resulted in a large outbreak in the Republic of Korea (South Korea) in 2015.[19]Lee SI. Costly lessons from the 2015 Middle East respiratory syndrome coronavirus outbreak in Korea. J Prev Med Public Health. 2015 Nov;48(6):274-6.
https://www.jpmph.org/journal/view.php?doi=10.3961/jpmph.15.064
http://www.ncbi.nlm.nih.gov/pubmed/26639740?tool=bestpractice.com
[20]Hui DS, Perlman S, Zumla A. Spread of MERS to South Korea and China. Lancet Respir Med. 2015 Jul;3(7):509-10.
http://www.ncbi.nlm.nih.gov/pubmed/26050550?tool=bestpractice.com
[21]Ha KM. A lesson learned from the MERS outbreak in South Korea in 2015. J Hosp Infect. 2016 Mar;92(3):232-4.
https://www.journalofhospitalinfection.com/article/S0195-6701(15)00397-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26601605?tool=bestpractice.com
Contact with infected dromedary camels is also a risk factor.[49]Alraddadi BM, Watson JT, Almarashi A, et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49-55.
https://wwwnc.cdc.gov/eid/article/22/1/15-1340_article
http://www.ncbi.nlm.nih.gov/pubmed/26692185?tool=bestpractice.com
Ninety-eight percent of cases have been reported in adults (defined as age >14 years).[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
Although infection has been reported in different age groups within the adult population, the median age of patients ranges from 50 to 67 years of age.[4]WHO MERS-CoV Research Group. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3828229
http://www.ncbi.nlm.nih.gov/pubmed/24270606?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
[27]Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090-5.
http://www.ncbi.nlm.nih.gov/pubmed/25278221?tool=bestpractice.com
Age ≥50 years is associated with more severe presentation, worse outcomes, and a higher risk of mortality.[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[10]Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-6.
http://www.ijidonline.com/article/S1201-9712(14)01622-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25303830?tool=bestpractice.com
Infection in children is rare, although the reason for this is unknown.[13]Memish ZA, Al-Tawfiq JA, Assiri A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014 Sep;33(9):904-6.
http://www.ncbi.nlm.nih.gov/pubmed/24763193?tool=bestpractice.com
[14]Khuri-Bulos N, Payne DC, Lu X, et al. Middle East respiratory syndrome coronavirus not detected in children hospitalized with acute respiratory illness in Amman, Jordan, March 2010 to September 2012. Clin Microbiol Infect. 2014 Jul;20(7):678-82.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61157-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/24313317?tool=bestpractice.com
Comorbid conditions, specifically diabetes mellitus, chronic renal impairment, heart disease, and obesity, are all risk factors for infection, as is smoking.[49]Alraddadi BM, Watson JT, Almarashi A, et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49-55.
https://wwwnc.cdc.gov/eid/article/22/1/15-1340_article
http://www.ncbi.nlm.nih.gov/pubmed/26692185?tool=bestpractice.com
Comorbid diabetes mellitus was reported in 65% to 87% of confirmed cases and has been associated with severe infection.[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
[27]Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090-5.
http://www.ncbi.nlm.nih.gov/pubmed/25278221?tool=bestpractice.com
Patients with end-stage renal disease were reported to develop severe infection and had worse clinical outcomes.[71]Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389-397.
http://annals.org/aim/article/1817260/clinical-course-outcomes-critically-ill-patients-middle-east-respiratory-syndrome
http://www.ncbi.nlm.nih.gov/pubmed/24474051?tool=bestpractice.com
Approximately 75% of patients with MERS have at least one comorbid illness.[4]WHO MERS-CoV Research Group. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3828229
http://www.ncbi.nlm.nih.gov/pubmed/24270606?tool=bestpractice.com
The WHO has developed a questionnaire designed to gather initial information about the potential exposures of a suspected or confirmed case in the 14 days before symptom onset:
World Health Organization (WHO): MERS-CoV - initial interview questionnaire of cases
Opens in new window
Clinical presentation
MERS may present in a similar way to the common cold. The majority of patients present with fever and respiratory symptoms (e.g., cough, dyspnoea).
Fever (temperature >38°C [100.4°F]): common symptom reported in 40% to 98% of cases.[7]Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013 Aug 1;369(5):407-16.
http://www.nejm.org/doi/full/10.1056/NEJMoa1306742#=article
http://www.ncbi.nlm.nih.gov/pubmed/23782161?tool=bestpractice.com
[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
[10]Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-6.
http://www.ijidonline.com/article/S1201-9712(14)01622-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25303830?tool=bestpractice.com
Fever may be absent in older patients, immunocompromised patients, pregnant women, and patients with end-stage renal disease, diabetes mellitus, or haemochromatosis; therefore, absence of fever should not preclude work-up for MERS.[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[72]Shalhoub S, Al-Zahrani A, Simhairi R, et al. Successful recovery of MERS CoV pneumonia in a patient with acquired immunodeficiency syndrome: a case report. J Clin Virol. 2015;62:69-71.
http://www.ncbi.nlm.nih.gov/pubmed/25542475?tool=bestpractice.com
Cough: common symptom reported in 54% to 86% of cases. It is usually dry; however, has been reported to be productive in 23% to 36% of patients.[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
[10]Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-6.
http://www.ijidonline.com/article/S1201-9712(14)01622-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25303830?tool=bestpractice.com
Dyspnoea: common symptom reported in 60% to 72% of cases.[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
[10]Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-6.
http://www.ijidonline.com/article/S1201-9712(14)01622-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25303830?tool=bestpractice.com
Haemoptysis: less common symptom reported in 7% to 17% of cases.[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
[10]Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-6.
http://www.ijidonline.com/article/S1201-9712(14)01622-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25303830?tool=bestpractice.com
Patients may also present with gastrointestinal symptoms:
Diarrhoea: reported in 7% to 26% of cases[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
Abdominal pain: reported in 17% to 24% of cases[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[10]Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-6.
http://www.ijidonline.com/article/S1201-9712(14)01622-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25303830?tool=bestpractice.com
Nausea/vomiting: reported in 7% to 21% of cases.[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
Patients may present with gastrointestinal symptoms only, going on to develop respiratory symptoms or pneumonia later in the course of infection. Other symptoms include myalgia, arthralgia, headache, chills/rigors, sore throat, and rhinorrhoea.[4]WHO MERS-CoV Research Group. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3828229
http://www.ncbi.nlm.nih.gov/pubmed/24270606?tool=bestpractice.com
[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[6]Oboho IK, Tomczyk SM, Al-Asmari AM, et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N Engl J Med. 2015 Feb 26;372(9):846-54.
http://www.nejm.org/doi/full/10.1056/NEJMoa1408636#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25714162?tool=bestpractice.com
[7]Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013 Aug 1;369(5):407-16.
http://www.nejm.org/doi/full/10.1056/NEJMoa1306742#=article
http://www.ncbi.nlm.nih.gov/pubmed/23782161?tool=bestpractice.com
[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[9]Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 Jul 15;59(2):160-5.
https://academic.oup.com/cid/article/59/2/160/2895452
http://www.ncbi.nlm.nih.gov/pubmed/24723278?tool=bestpractice.com
[10]Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-6.
http://www.ijidonline.com/article/S1201-9712(14)01622-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25303830?tool=bestpractice.com
Some patients, particularly young, healthy patients, may be asymptomatic or present with mild respiratory symptoms and a normal chest x-ray.[11]Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013 Aug 29;369(9):884-6.
https://www.nejm.org/doi/10.1056/NEJMc1308698
http://www.ncbi.nlm.nih.gov/pubmed/23923992?tool=bestpractice.com
However, others, particularly older patients or those with comorbidities, may present with severe, rapidly progressive pneumonia, acute respiratory distress syndrome, septic shock, or multi-organ failure resulting in death.
Pneumonia is a common finding, but not always present. Rapid progression to pneumonia can occur in less than a week. Crackles/rales and bronchial breath sounds may be noted on auscultation. Chest pain, dyspnoea, tachypnoea, tachycardia, and cyanosis may be present.
Case definitions
Case definitions have been published by the WHO and the Ministry of Health (Saudi Arabia).[73]Ministry of Health (Saudi Arabia). Middle East respiratory syndrome coronavirus; guidelines for healthcare professionals, version 5.1. May 2018 [internet publication].
https://www.moh.gov.sa/CCC/healthp/regulations/Documents/MERS-CoV%20Guidelines%20for%20Healthcare%20Professionals%20-%20May%202018%20-%20v5.1%20%281%29.pdf
[74]World Health Organization (WHO). Middle East respiratory syndrome coronavirus: case definition for reporting to WHO. July 2017 [internet publication].
https://www.who.int/csr/disease/coronavirus_infections/mers-interim-case-definition.pdf?ua=1
Because MERS is considered an emerging disease, definitions are constantly evolving and not all clinical presentations will fit the case definitions. Physicians should be vigilant for identifying suspected cases regardless of whether they fit the case definitions or not. For example, the absence of fever has been reported in cases of confirmed infection, despite most case definitions including fever as a prerequisite for diagnosis.
Current case definitions:
Initial laboratory investigations
Laboratory testing is recommended in any patient who presents with symptoms such as fever, respiratory symptoms, gastrointestinal symptoms, and/or myalgia in the correct epidemiological context.
FBC commonly reveals leukopenia, lymphopenia, and thrombocytopenia.[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[27]Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090-5.
http://www.ncbi.nlm.nih.gov/pubmed/25278221?tool=bestpractice.com
[71]Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389-397.
http://annals.org/aim/article/1817260/clinical-course-outcomes-critically-ill-patients-middle-east-respiratory-syndrome
http://www.ncbi.nlm.nih.gov/pubmed/24474051?tool=bestpractice.com
Patients may have leukocytosis, particularly in the setting of a secondary bacterial infection. Specific diagnostic testing for MERS should be pursued even in the absence of a typical FBC result.
A comprehensive metabolic panel should also be ordered, and may show elevated creatinine, liver function tests, and lactate dehydrogenase.[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
[27]Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090-5.
http://www.ncbi.nlm.nih.gov/pubmed/25278221?tool=bestpractice.com
[71]Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389-397.
http://annals.org/aim/article/1817260/clinical-course-outcomes-critically-ill-patients-middle-east-respiratory-syndrome
http://www.ncbi.nlm.nih.gov/pubmed/24474051?tool=bestpractice.com
Blood cultures should be collected to test for potential bacterial pathogens that can also cause pneumonia or sepsis.[75]World Health Organization. Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus (MERS-CoV) infection is suspected. Interim guidance. Jan 2019 [internet publication].
https://www.who.int/publications/i/item/WHO-MERS-Clinical-15-1-Revision-1
They should be collected before empirical antimicrobial therapy is started, if possible.
The CDC and WHO have produced detailed guidance on laboratory testing:
Molecular testing
All patients with suspected MERS should undergo molecular testing as soon as possible after onset of illness. The CDC recommends testing in patients in the US if they have an illness consistent with certain defined clinical features and epidemiological risk criteria.[76]Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS): diagnostic testing for MERS. Dec 2024 [internet publication].
https://www.cdc.gov/mers/hcp/diagnosis-testing/index.html
Confirmation of infection is based on the detection of unique sequences of viral RNA by real-time reverse transcription polymerase chain reaction (RT-PCR), with confirmation by nucleic acid sequencing if necessary.
Lower respiratory tract specimens (e.g., sputum, tracheal aspirates, bronchoalveolar lavage fluid) are the preferred specimen for RT-PCR as sputum and tracheal aspirates contain the highest viral loads, and hence have the highest yield.[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[77]Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590-1594.
https://academic.oup.com/jid/article/210/10/1590/2192931/Respiratory-Tract-Samples-Viral-Load-and-Genome
http://www.ncbi.nlm.nih.gov/pubmed/24837403?tool=bestpractice.com
However, bronchoscopy may generate aerosols and is generally not recommended. Upper respiratory tract specimens (e.g., nasopharyngeal and oropharyngeal swabs, nasopharyngeal aspirate/wash) and serum collection for virus detection are recommended, especially if lower respiratory specimens are not available and it is 7 to 10 days since symptom onset.[78]Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021 Jan;2(1):e13-e22.
https://www.doi.org/10.1016/S2666-5247(20)30172-5
http://www.ncbi.nlm.nih.gov/pubmed/33521734?tool=bestpractice.com
Both upper and lower respiratory tract specimens should be collected whenever possible.[73]Ministry of Health (Saudi Arabia). Middle East respiratory syndrome coronavirus; guidelines for healthcare professionals, version 5.1. May 2018 [internet publication].
https://www.moh.gov.sa/CCC/healthp/regulations/Documents/MERS-CoV%20Guidelines%20for%20Healthcare%20Professionals%20-%20May%202018%20-%20v5.1%20%281%29.pdf
[79]World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus. Interim guidance (revised). January 2018 [internet publication].
https://www.who.int/publications/i/item/10665-259952
[80]Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS): laboratory testing for MERS. Dec 2024 [internet publication].
https://www.cdc.gov/coronavirus/mers/lab/lab-testing.html
Urine and stool specimens may also be used; however, these specimens contain lower levels of the virus compared with respiratory tract specimens. Healthcare workers should wear appropriate personal protective equipment (e.g., mask, eye protection, gloves, gown) when collecting specimens.[79]World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus. Interim guidance (revised). January 2018 [internet publication].
https://www.who.int/publications/i/item/10665-259952
There are three RT-PCR assays currently recommended for the diagnosis of MERS-CoV infection:[79]World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus. Interim guidance (revised). January 2018 [internet publication].
https://www.who.int/publications/i/item/10665-259952
MERS-CoV RT-PCR (upE): highly sensitive screening assay targeting regions upstream of the E protein gene (upE)
MERS-CoV RT-PCR (ORF 1b): confirmatory assay targeting the open reading frame 1b (ORF 1b). It is less sensitive than the upE assay, but is more specific as it does not exhibit cross-reactivity with the five main coronaviruses known to infect humans (i.e., OC43, NL63, 229E, SARS, coronavirus disease 2019 [COVID-19])
MERS-CoV RT-PCR (ORF 1a): confirmatory assay targeting the open reading frame 1a (ORF 1a). It is highly specific and more sensitive than the ORF 1b assay, but has similar sensitivity to the upE assay.
Assays targeting sequencing amplicons on the viral genome are also available and can aid confirmation of the diagnosis. An assay targeting the RdRp gene (RdRpSeq) broadly detects betacoronavirus clade C sequences; however, it is not specific and will detect other coronavirus strains including human coronaviruses HKU1 and OC43.[81]Corman VM, Muller MA, Costabel U, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:20334.
http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20334
http://www.ncbi.nlm.nih.gov/pubmed/23231891?tool=bestpractice.com
Another assay targets N gene sequencing (NSeq). This region was chosen as it comprised a 2 amino acid deletion in the corresponding sequence published from a patient treated in the UK. It is highly sensitive and specific for detection of human coronavirus Erasmus Medical Center/2012 (hCoV-EMC), the strain isolated from the first person infected with MERS.[81]Corman VM, Muller MA, Costabel U, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:20334.
http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20334
http://www.ncbi.nlm.nih.gov/pubmed/23231891?tool=bestpractice.com
Both of these assays are sensitive enough to detect the virus at very low concentrations, but if used should be coupled with a subsequent confirmatory assay.
The WHO recommends a screening assay to be performed first and, if positive, a confirmatory assay should be performed. If the confirmatory assay is positive, infection is confirmed. If the confirmatory assay is negative, consider repeating the tests (if epidemiological evidence is suggestive of infection) or perform sequencing assays. If sequencing indicates the presence of MERS-CoV, infection is confirmed.[79]World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus. Interim guidance (revised). January 2018 [internet publication].
https://www.who.int/publications/i/item/10665-259952
[Figure caption and citation for the preceding image starts]: Algorithm for testing cases under investigation by reverse transcription polymerase chain reaction (RT-PCR)Produced by the BMJ Evidence Centre (adapted from WHO laboratory testing for Middle East respiratory syndrome coronavirus -interim guidance (revised) WHO/MERS/LAB/15.1/Rev1/2018) [Citation ends].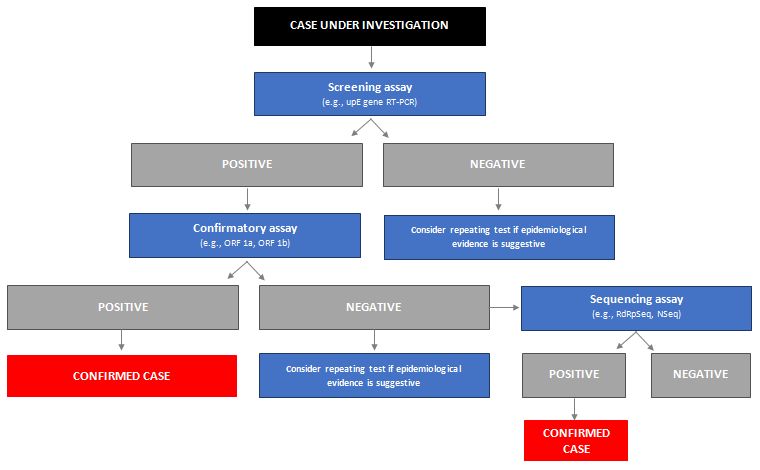
The WHO and CDC defines a confirmed case as a person with laboratory confirmation of infection.[74]World Health Organization (WHO). Middle East respiratory syndrome coronavirus: case definition for reporting to WHO. July 2017 [internet publication].
https://www.who.int/csr/disease/coronavirus_infections/mers-interim-case-definition.pdf?ua=1
[82]Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS): managing MERS cases and contacts. Dec 2024 [internet publication].
https://www.cdc.gov/mers/php/contact-tracing/index.html
Presence of nucleic acid can be confirmed by either a positive RT-PCR result on at least two specific genomic targets or a single positive target with sequencing of a second target.[74]World Health Organization (WHO). Middle East respiratory syndrome coronavirus: case definition for reporting to WHO. July 2017 [internet publication].
https://www.who.int/csr/disease/coronavirus_infections/mers-interim-case-definition.pdf?ua=1
False-negative results can occur due to poor specimen quality, incorrect handling of the specimen, or the time of collection; therefore, in patients who test negative where there is a high index of suspicion for infection, additional specimens should be collected (preferably lower respiratory tract specimens) and tested.
Serological testing
Serological testing is generally not used for diagnosis, but for epidemiological surveillance or investigational purposes (e.g., retrospective diagnosis). It may be used to confirm the diagnosis; however, a single specimen would only identify a probable case. Paired sampling taken at least 14 to 21 days apart is required to confirm the diagnosis.
Several serological tests have been developed for detecting MERS-CoV antibodies including an indirect fluorescent antibody (IFA) test, an enzyme-linked immunosorbent assay (ELISA), and a serum neutralisation test.
The WHO defines a confirmed case to be a patient with evidence of seroconversion in at least one screening assay (e.g., IFA, ELISA) and confirmation by a neutralisation assay in samples taken at least 14 days apart. They define a probable case as a symptomatic patient without a positive RT-PCR test who has a positive result for at least one screening assay (e.g., IFA, ELISA) plus a positive result for a neutralisation assay in a single specimen.[79]World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus. Interim guidance (revised). January 2018 [internet publication].
https://www.who.int/publications/i/item/10665-259952
False-positive results can occur due to cross-reactivity with other betacoronaviruses.
Imaging
A chest x-ray should be ordered in all patients with suspected pneumonia. Diffuse bilateral infiltrates have been reported in 22% to 67% of cases. Lobar infiltrates or the absence of infiltrates have also been reported, particularly in healthy, young patients.[5]Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha-2a or IFN-beta-1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32.
http://www.ncbi.nlm.nih.gov/pubmed/25900158?tool=bestpractice.com
[8]Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752-61.
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(13)70204-4/fulltext#seccestitle10
http://www.ncbi.nlm.nih.gov/pubmed/23891402?tool=bestpractice.com
A CT scan of the chest may be helpful in patients with suspected pneumonia who have a normal chest x-ray. CT may reveal bilateral subpleural and basal airspace opacities, with more extensive ground-glass opacities than consolidation.[83]Ajlan AM, Ahyad RA, Jamjoom LG, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203:782-787.
http://www.ajronline.org/doi/full/10.2214/AJR.14.13021
http://www.ncbi.nlm.nih.gov/pubmed/24918624?tool=bestpractice.com
Recognition of these patterns can aid early diagnosis of MERS; however, routine use of this test is not recommended.
