Approach
HIT should be suspected when a patient presents with new thrombocytopenia and/or thrombosis in the context of confirmed or suspected exposure to heparin (i.e., unfractionated heparin, low molecular weight heparin [LMWH], or, more rarely, fondaparinux) within the past 100 days, particularly following recent cardiac or orthopaedic surgery. HIT should also be considered when a patient presents with adrenal haemorrhagic necrosis (secondary to adrenal vein thrombosis), necrotising skin lesions at heparin injection sites, or an acute systemic reaction in the context of exposure to heparin or LMWH within the past 100 days.
Diagnosis requires the combination of both a compatible clinical picture and laboratory confirmation of the presence of heparin-dependent platelet-activating HIT antibodies. The presence of HIT antibodies alone, without any clinical manifestations, is not sufficient for a diagnosis of HIT.
Clinical picture
The first step is to determine the likelihood that a patient has HIT based on clinical criteria. This includes a careful review of the patient's history of heparin exposure (i.e., unfractionated heparin, LMWH, or fondaparinux). The risk of HIT, according to heparin type, in order from highest to lowest is as follows: unfractionated heparin >LMWH >fondaparinux.[16][32]
[  ]
Fondaparinux, a pentasaccharide anticoagulant, does not usually promote antibody binding to platelet factor 4 (PF4), despite its structural similarity to heparin, owing to absent/weak cross-reactivity. Therefore, fondaparinux has a very low risk of inducing HIT. Observational studies report the successful use of fondaparinux to treat HIT, and it is considered to be a non-heparin anticoagulant.[2][3]
]
Fondaparinux, a pentasaccharide anticoagulant, does not usually promote antibody binding to platelet factor 4 (PF4), despite its structural similarity to heparin, owing to absent/weak cross-reactivity. Therefore, fondaparinux has a very low risk of inducing HIT. Observational studies report the successful use of fondaparinux to treat HIT, and it is considered to be a non-heparin anticoagulant.[2][3]
An absence of conditions or medications that cause thrombocytopenia increases the likelihood of HIT. A history of recent surgery (especially orthopaedic or cardiovascular) or trauma increases the likelihood of HIT in patients exposed to heparin. Patients with a history of HIT who are re-exposed to heparin for at least 4 days are at risk of recurrence.
Features consistent with a recent venous or arterial thromboembolic event are common (e.g., deep vein thrombosis (DVT), pulmonary embolism [PE], stroke, myocardial infarction [MI]). These include: new unilateral leg oedema, tenderness, or discoloration (DVT); chest pain, tachypnoea, hypotension, or tachycardia (PE or MI); focal neurological deficits (stroke).
Fever, chills, tachycardia, hypertension, dyspnoea, or cardiopulmonary arrest may occur within 30 minutes of a dose of heparin and is usually accompanied by an abrupt drop in platelet count.
Necrosis may be seen on examination at the heparin injection sites. Bleeding in patients with HIT is rare and there may be no or minimal signs of bleeding (e.g., petechiae, ecchymosis). Patients with adrenal haemorrhagic necrosis may present with abdominal pain, refractory hypotension, and Addisonian crisis. Patients with cerebral venous thrombosis may present with headache, nausea, vomiting, and/or neurological deficits. Rarely, patients with HIT-provoked DVT will present with venous limb gangrene (as a consequence of inappropriate treatment with a vitamin K antagonist).[7][Figure caption and citation for the preceding image starts]: Venous limb gangrene of left foot in HIT: (A) dorsal aspect; (B) medial aspect; and (C) plantar aspectRozati H, Shah SP, Peng YY. Lower limb gangrene postcardiac surgery. BMJ Case Reports. 2013; doi:10.1136/bcr-2012-008362 [Citation ends].
Clinical prediction tools
A number of clinical prediction tools have been developed to help physicians determine clinical probability of HIT (e.g., 4Ts score, HIT expert probability [HEP] score).
While the clinical prediction rules differ on specific details, they all focus on several key features:
Magnitude of the platelet count fall
Timing of the platelet count fall (or other HIT-related event) in relation to start of heparin
Presence or absence of alternative explanations for thrombocytopenia.
The 4Ts score has been subject to greater evaluation than other tools, and is recommended by the American Society of Hematology:[36][37][38][39][40]
Points from 0-2 are given for 4 categories: magnitude of Thrombocytopenia, Timing of onset of platelet fall (or other sequelae of HIT), Thrombosis, and oTher explanation for the platelet fall.
Pre-test probability of HIT:
A low score (0-3) indicates <1% probability of HIT
An intermediate score (4-5) indicates approximately 10% probability of HIT
A high score (6-8) indicates approximately 50% probability of HIT.
A typical high 4Ts score would be a patient who experiences a 50% drop in platelet count with a nadir ≥20 × 10⁹/L (>20 × 10³/microlitre) between days 5 and 10 of heparin exposure, and is found to have new thrombosis and no alternative explanation for the drop in platelet count.
HEP score:[41]
Points from -3 to +3 are given for 8 categories: magnitude of fall in platelet count, timing of fall in platelet count, nadir platelet count, thrombosis, skin necrosis, acute systemic reaction, bleeding, other causes of thrombocytopenia.
Laboratory confirmation of HIT
A full blood count (FBC) should be ordered in all patients with suspected HIT and typically shows a falling platelet count. The timing of the fall in platelet count (beginning from the first day of heparin exposure [day 0]) is key. An example would be a patient who experiences a 50% drop in platelet count with a nadir ≥20 × 10⁹/L (>20 × 10³/microlitre) between days 5 and 10 of heparin exposure. The platelet count does not have to fall below 150 × 10⁹/L (150 × 10³/microlitre) for HIT to be considered (e.g., 50% or more decrease from baseline during the correct time frame is still suspicious for HIT, even if the absolute platelet nadir is >150 × 10⁹/L [>150 × 10³/microlitre]).
It is not uncommon for the platelet count to initially fall after surgery and then rise to a level higher than the preoperative count (rebound thrombocytosis). In such cases, the postoperative rebound platelet count should be considered the new baseline count in these patients when determining the clinical probability of HIT. Thrombocytopenia in the context of pancytopenia reduces the likelihood of HIT.
Coagulation studies (i.e., international normalised ratio, activated partial thromboplastin time) should be ordered in patients with suspected HIT to exclude coagulopathy. HIT may induce disseminated intravascular coagulation in 10% to 20% of patients; therefore, coagulopathy and low fibrinogen levels do not exclude HIT if the clinical scenario is otherwise consistent.[42]
Patients with at least an intermediate clinical suspicion for HIT (i.e., 4Ts score of ≥4) should undergo testing for HIT antibodies.[38] A low 4Ts score (i.e., ≤3) alone has high negative predictive value; guidelines recommend against laboratory testing in these patients.[38][43][44] If, however, there is uncertainty about the score (e.g., multiple missing platelet counts, history of recent heparin exposure is unclear, concurrent potential causes of thrombocytopenia), testing for HIT should be considered.[38][45]
A wide variety of laboratory assays are used to confirm the presence of HIT antibodies. These assays generally fall into one of two categories:
Antigen assays (e.g., anti-platelet factor 4 [PF4]/H enzyme-linked immunosorbent assay [ELISA], H/PF4-PaGIA) are available at most clinical centres. Antigen assays are highly sensitive (>99%), but they have a high false-positive rate. False positives result from detection of all types of HIT antibodies, regardless of their ability to activate platelets.[46] For example, up to 50% of cardiovascular surgery patients will develop HIT antibodies, but only 2% of those patients will develop HIT.[11] ELISAs that detect IgG antibodies only are more specific for HIT and the higher the titre of the antigen assay, the higher the likelihood the patient has platelet-activating antibodies (i.e., the greater the likelihood the patient has HIT or improved specificity).[36] Some rapid immunoassays that can provide results in less than 30 minutes appear to have similar diagnostic properties to the ELISAs.[45][47]
Functional assays (e.g., serotonin release assay, heparin-induced platelet activation) are limited to a small number of clinical centres, but have better specificity than the antigen assays. Functional assays detect antibodies based on their platelet‐activating properties (i.e., antibodies that are more likely to be clinically significant).[48] These assays have high sensitivity (>95%) and specificity (>95%) for HIT; therefore, in the context of a compatible clinical picture, a positive result confirms HIT and a negative result excludes HIT.[10][49]
As many centres do not have access to functional assays, diagnosis is often based on clinical suspicion for HIT (based on the 4Ts score) combined with antigen assay results, as shown in the table below.[50]
[Figure caption and citation for the preceding image starts]: Combination of clinical picture and laboratory evidence of HIT antibodiesCreated by the BMJ Knowledge Centre based on information from: Raschke RA, Gallo T, Curry SC, et al. Clinical effectiveness of a Bayesian algorithm for the diagnosis and management of heparin-induced thrombocytopenia. J Thromb Haemost. 2017 Aug;15(8):1640-5. [Citation ends].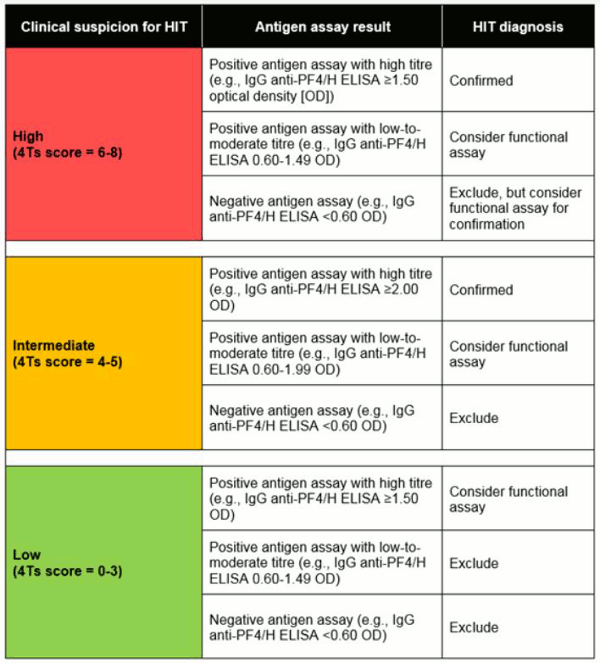
Imaging
Venous Doppler ultrasound should be ordered in patients with suspected DVT. New DVT (incompressible venous segment) or extension of a recent DVT (incompressible venous segment previously fully compressible) increases the likelihood of HIT.[51]
Computed tomography pulmonary angiogram (CTPA) or ventilation-perfusion scan (V/Q scan) should be performed in patients with suspected pulmonary embolism, and a cerebral computed tomography venogram (CTV) or magnetic resonance venography (MRV) of the head in patients with suspected cerebral venous thrombosis.[51]
Thrombosis has been reported in up to 50% of patients with untreated HIT.[52] In the context of confirmed HIT, the presence of a DVT may lengthen the duration of treatment.
Use of this content is subject to our disclaimer