Aetiology
Causes can be divided into those that are central (gonadotrophin-dependent), in which there is premature activation of the hypothalamo-pituitary-gonadal axis; and those due to premature activation of the ovaries, testes, or adrenal glands independent of gonadotrophin secretion (gonadotrophin-independent precocious puberty [GIPP]).
Central precocious puberty (CPP)
Idiopathic CPP is the most common cause in females (97% in girls 6-8 years and 75% in girls <6 years).[23] In males, the reported incidence of idiopathic CPP is more variable, ranging from 60% to around 90%.[24][25][26]
Brain neoplasms such as optic nerve gliomas (e.g., in association with neurofibromatosis), craniopharyngiomas, or hamartomas.[27][28] Hamartomas of the tuber cinereum are congenital tumours composed of a heterotopic mass including gonadotrophin-releasing hormone (GnRH) neurosecretory neurons, frequently associated with CPP, and often occur before 3 years of age, particularly in males. Other tumours include astrocytomas, ependymomas, pineal tumours.
Cranial radiotherapy.[29]
Neurodisability conditions such as hydrocephalus, cerebral palsy, post-infection such as meningitis or encephalitis.[30]
Post-traumatic head injury.[30]
Gain-of-function mutations in KISS1R, a G protein-coupled receptor that is a ligand for kisspeptin, can cause CPP, although these mutations are found very rarely.[31] The interaction between KISS1R and kisspeptin is necessary for GnRH function. More commonly, particularly in familial CPP, mutations in the MKRN3 gene are found as the cause. MKRN3 is an imprinted gene whose function is to suppress the hypothalamic-pituitary-gonadal axis before puberty.[32] Mutations in DLK1, a second imprinted gene, are a further rare genetic cause of CPP.[33]
Midline forebrain abnormalities such as holoprosencephaly or septo-optic dysplasia.[34]
Association with child adoption and sexual abuse.[35][36][37]
Gonadotrophin-independent precocious puberty (GIPP)
Ovarian causes: follicular cysts of the ovary, granulosa cell tumours, Leydig cell tumours, and gonadoblastoma.
Testicular causes: Leydig cell tumours and a defect of luteinising hormone (LH) receptor function (testotoxicosis or familial GIPP). The latter is caused by an activating mutation in the LH receptor gene.
Adrenal causes: 21-hydroxylase and, less commonly, 11-hydroxylase deficiency congenital adrenal hyperplasia (CAH) in males results in GIPP. CAH in females presents with signs of virilisation (e.g., pubic and axillary hair and clitoromegaly) but no breast development. Other adrenal causes include Cushing's syndrome and an adrenal virilising tumour. Prolonged sex steroid exposure in GIPP has a direct maturational effect on the hypothalamus and can accelerate the onset of CPP.
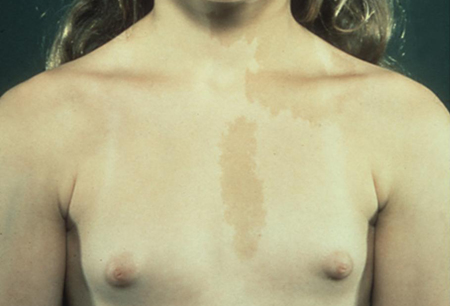
McCune-Albright syndrome (MAS), a sporadic condition caused by a somatic activating missense mutation in the gene encoding the alpha-subunit of the G-protein that stimulates cyclic AMP production. It results in the classic triad of GIPP, café au lait spots, and fibrous dysplasias of the bone. Precocious puberty due to MAS is much more common in girls than in boys. Autonomous hyperfunctioning most commonly involves the ovaries, but other endocrine involvement includes the thyroid (thyrotoxicosis), adrenals (Cushing's syndrome), pituitary (gigantism/acromegaly or hyperprolactinaemia), and parathyroid glands (hyperparathyroidism).[Figure caption and citation for the preceding image starts]: Female with gonadotrophin-independent precocious puberty (GIPP) and café au lait hyperpigmented macules in McCune-Albright SyndromeFrom the collection of Dr A. Mehta; used with permission [Citation ends].
Exposure to exogenous hormones such as the contraceptive pill or testosterone gels may be responsible for early pubertal development in rare cases. Oestrogenic agents in cosmetics and food products have also been implicated in causing an earlier age of puberty; 'epidemics' of premature thelarche (isolated breast development) in some geographic areas may be linked to an environmental exposure to oestrogens.[22]
Human chorionic gonadotrophin-secreting germ cell tumours are a rare cause in males only.[38] These tumours may occur in the gonads, brain (usually in the pineal region), liver, retroperitoneum, and posterior mediastinum.
Pathophysiology
A nocturnal increase in the amplitude and pulsatility of gonadotrophin-releasing hormone (GnRH) secretion from the hypothalamus is the first change evident at puberty.[39] GnRH release is the predominant determinant of progression into and through puberty. It follows a period of quiescence from approximately 2 years of age to 8 to 9 years. Normal GnRH function depends on the interaction between inhibitory (e.g., gamma-aminobutyric acid) and excitatory (e.g., kisspeptin, glutamate) neuronal glial inputs.[40]
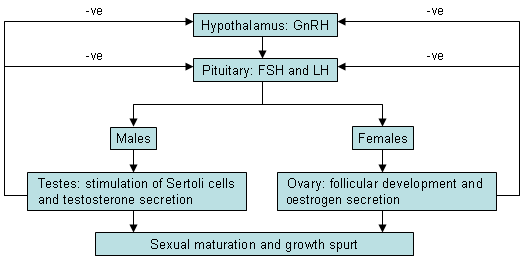
Hypothalamic GnRH in turn stimulates the secretion of pituitary hormones: follicle-stimulating hormone (FSH) and luteinising hormone (LH). In males, LH acts on the Leydig cells to produce testosterone, while FSH acts on the Sertoli cells to enhance spermatogenesis. In females, a complex interaction between LH and FSH on the thecal and granulosa cells results in follicular development with oestrogen production and ovulation.
In centrally mediated precocious puberty when the hypothalamo-pituitary-gonadal axis is prematurely activated, the serum gonadotrophins are elevated (gonadotrophin-dependent precocious puberty). The pattern of endocrine change is the same as in normal puberty (i.e., it is consonant).
In precocious puberty due to other causes (adrenal, testicular, ovarian, or exogenous hormones), the secretion of sex steroids is autonomous and independent of the hypothalamic GnRH pulse. There is loss of normal feedback regulation and sex steroid concentrations are elevated, typically with low or suppressed gonadotrophins. This is called gonadotrophin-independent precocious puberty (GIPP). Pubertal development does not follow the pattern of normal puberty (i.e., it is disconsonant). Prolonged sex-steroid exposure has a direct maturational effect on the hypothalamus and can accelerate the onset of centrally mediated puberty.[Figure caption and citation for the preceding image starts]: Hypothalamo-pituitary-gonadal feedback loop. GnRH, gonadotrophin-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinising hormoneFrom the collection of Dr A. Mehta; used with permission [Citation ends].
On average, puberty takes approximately 2 to 5 years to complete and provides a growth increment of 25 cm in girls and 30 cm in boys. Oestrogen, either from the ovary or aromatised from testicular testosterone, is the factor that mediates the increased growth hormone response during puberty.[41][42] The premature secretion of gonadal steroids in children results in the tall stature during childhood, due to an accelerated rate of linear growth, with normal or short stature as an adult, due to early fusion of the epiphyseal growth plates.
Classification
Etiological classification
Central precocious puberty (CPP)
In CPP (gonadotrophin-dependent precocious puberty), the hypothalamo-pituitary-gonadal axis is prematurely activated.
The pattern of endocrine change is the same as in normal puberty (i.e., pubertal development is consonant).
Gonadotrophin-independent precocious puberty (GIPP)
The secretion of sex steroids (oestrogen or testosterone) is autonomous and independent of the central hypothalamic gonadotrophin-releasing hormone (GnRH) pulse.
There is loss of normal feedback regulation and sex steroid concentrations are typically elevated with low levels of gonadotrophins.
Pubertal development does not follow the pattern of normal puberty (i.e., it is disconsonant).
Prolonged sex steroid exposure in GIPP has a direct maturational effect on the hypothalamus and can accelerate the onset of CPP.
Use of this content is subject to our disclaimer