The major manifestations of AAT deficiency are hepatic and pulmonary.
Panacinar emphysema and associated obstructive lung disease are the most common manifestations.[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
[39]Marciniuk DD, Hernandez P, Balter M, et al. Alpha-1 antitrypsin deficiency targeted testing and augmentation therapy: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2012;19:109-116.
https://www.hindawi.com/journals/crj/2012/920918
http://www.ncbi.nlm.nih.gov/pubmed/22536580?tool=bestpractice.com
Evidence suggests that almost 60% of patients develop severe pulmonary disease.[24]Larsson C. Natural history and life expectancy in severe alpha 1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345-351.
http://www.ncbi.nlm.nih.gov/pubmed/309708?tool=bestpractice.com
Bronchiectasis may occur. Up to 95% of patients with PI*ZZ AAT deficiency have radiological evidence of bronchiectasis (but PI*ZZ AAT deficiency has been found in <1% of patients who present with bronchiectasis).[40]Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019 Jan;74(suppl 1):1-69.
https://thorax.bmj.com/content/74/Suppl_1/1.long
http://www.ncbi.nlm.nih.gov/pubmed/30545985?tool=bestpractice.com
Liver disease usually initially presents as hepatitis and jaundice, although severe disease may progress to cirrhosis and hepatocellular carcinoma. Liver involvement may also be seen in neonates with AAT deficiency.[9]Lopes AP, Mineiro MA, Costa F, et al. Portuguese consensus document for the management of alpha-1-antitrypsin deficiency. Pulmonology. 2018 Dec;24 Suppl 1:1-21.
https://www.doi.org/10.1016/j.pulmoe.2018.09.004
http://www.ncbi.nlm.nih.gov/pubmed/30473034?tool=bestpractice.com
[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
Necrotising panniculitis and granulomatosis with polyangiitis are infrequent complications.
Evidence suggests that AAT deficiency may be under-recognised by physicians as a cause of lung and liver disease.[41]Silverman EK, Miletich JP, Pierce JA, et al. Alpha-1 antitrypsin deficiency: high prevalence in the St. Louis area determined by direct population screening. Am Rev Respir Dis. 1989;140:961-966.
http://www.ncbi.nlm.nih.gov/pubmed/2679271?tool=bestpractice.com
Further evidence demonstrates a significant time difference between the onset of clinical disease and diagnosis, with evaluation by multiple physicians in the interim.[42]Stoller JK, Smith P, Yang P, et al. Physical and social impact of alpha-1 antitrypsin deficiency: results of a survey. Cleve Clin J Med. 1994;61:461-467.
http://www.ncbi.nlm.nih.gov/pubmed/7828337?tool=bestpractice.com
Guidelines recommend a high clinical suspicion and quantitative AAT measurement in the following scenarios:[6]American Thoracic Society/European Respiratory Society Statement. Standards for the diagnosis and management of individuals with alpha 1-antitrypsin deficiency. Am J Respir Crit Care Med. 2003 Oct 1;168(7):818-900.
https://www.atsjournals.org/doi/full/10.1164/rccm.168.7.818
http://www.ncbi.nlm.nih.gov/pubmed/14522813?tool=bestpractice.com
[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
[43]World Health Organization. Alpha-1 antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Organ. 1997;75:397-415.
http://www.ncbi.nlm.nih.gov/pubmed/9447774?tool=bestpractice.com
[44]Eriksson S, Carlson J, Velez R. Risks for cirrhosis and primary liver cancer in alpha-1 antitrypsin deficiency. N Engl J Med. 1986;314:736-739.
http://www.ncbi.nlm.nih.gov/pubmed/3485248?tool=bestpractice.com
Airflow obstruction partially reversible or irreversible with bronchodilators
All patients with COPD[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
[45]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of COPD: 2025 report. 2024 [internet publication].
https://goldcopd.org/2025-gold-report
All patients with adult-onset asthma[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
A personal or family history of c-ANCA vasculitis (granulomatosis with polyangiitis is an infrequent complication of AAT deficiency)
Liver disease of unknown aetiology
Bronchiectasis of unknown aetiology, especially when co-existing with panacinar emphysema[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
[40]Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019 Jan;74(suppl 1):1-69.
https://thorax.bmj.com/content/74/Suppl_1/1.long
http://www.ncbi.nlm.nih.gov/pubmed/30545985?tool=bestpractice.com
Panniculitis of unknown aetiology (necrotising panniculitis is an infrequent complication of AAT deficiency)
Adolescents and adults with a sibling who is AAT homozygous
Asymptomatic people with persistent obstructive pulmonary dysfunction who report smoking or occupational exposure.
Historical factors
Presenting symptoms of pulmonary manifestations are non-specific and may include shortness of breath, shortness of breath on exertion, fatigue, wheezing, cough, and/or chest tightness.
Presenting symptoms of hepatic manifestations are non-specific and may include yellowing of the skin, fatigue, bleeding, bruising, abdominal distension, abdominal pain, and/or confusion.
It is important to consider age, occupation, and smoking history in patients with symptomatic lung disease, as these factors may point to AAT deficiency. The greatest risk factor for emphysema in patients with the PI*ZZ phenotype is smoking. Lung function and survival are both affected.[24]Larsson C. Natural history and life expectancy in severe alpha 1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345-351.
http://www.ncbi.nlm.nih.gov/pubmed/309708?tool=bestpractice.com
[46]Janus ED, Phillips NT, Carrell RW. Smoking, lung function, and alpha-1-antitrypsin deficiency. Lancet. 1985;1:152-4.
http://www.ncbi.nlm.nih.gov/pubmed/2857224?tool=bestpractice.com
[47]Wu MC, Eriksson S. Lung function, smoking and survival in severe alpha 1-antitrypsin deficiency, PiZZ. J Clin Epidemiol. 1988;41:1157-1165.
http://www.ncbi.nlm.nih.gov/pubmed/3264848?tool=bestpractice.com
However, some evidence suggests that ex-smokers and people who have never smoked have similar declines in lung function over time, and some smokers may never develop pulmonary symptoms.[42]Stoller JK, Smith P, Yang P, et al. Physical and social impact of alpha-1 antitrypsin deficiency: results of a survey. Cleve Clin J Med. 1994;61:461-467.
http://www.ncbi.nlm.nih.gov/pubmed/7828337?tool=bestpractice.com
[48]Piitulainen E, Eriksson S. Decline in FEV1 related to smoking status in individuals with severe alpha-1 antitrypsin deficiency (PiZZ). Eur Respir J. 1999;13:247-251.
http://erj.ersjournals.com/cgi/reprint/13/2/247
http://www.ncbi.nlm.nih.gov/pubmed/10065663?tool=bestpractice.com
Occupational or other exposure to gas, fumes, and/or dust has also been associated with decreased pulmonary function in patients with PI*ZZ AAT deficiency. This includes passive smoking and work with kerosene heaters.[49]Piitulainen E, Tornling G, Eriksson S. Effect of age and occupational exposure to airway irritants on lung function in non-smoking individuals with alpha-1 antitrypsin deficiency (PiZZ). Thorax. 1997;52:244-248.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1758519/pdf/v052p00244.pdf
http://www.ncbi.nlm.nih.gov/pubmed/9093340?tool=bestpractice.com
[50]Piitulainen E, Tornling G, Eriksson S. Environmental correlates of impaired lung function in non-smokers with severe alpha-1 antitrypsin deficiency (PiZZ). Thorax. 1998;53:939-943.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1745103/pdf/v053p00939.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10193391?tool=bestpractice.com
[51]Silverman EK, Pierce JA, Province MA, et al. Variability of pulmonary function in alpha-1 antitrypsin deficiency: clinical correlates. Ann Intern Med. 1989;111:982-991.
http://www.ncbi.nlm.nih.gov/pubmed/2596778?tool=bestpractice.com
[52]Piitulainen E, Sveger T. Effect of environmental and clinical factors on lung function and respiratory symptoms in adolescents with alpha-1 antitrypsin deficiency. Acta Paediatr. 1998;87:1120-1124.
http://www.ncbi.nlm.nih.gov/pubmed/9846912?tool=bestpractice.com
There is limited evidence to suggest that symptomatic lung disease is more prevalent in PI*ZZ males than in PI*ZZ females. However, this result is likely to be confounded by other variables, such as smoking and occupational exposure.[20]Kueppers F, Fallat R, Larson RK. Obstructive lung disease and alpha-1 antitrypsin deficiency gene heterozygosity. Science. 1969;165:899-901.
http://www.ncbi.nlm.nih.gov/pubmed/5816326?tool=bestpractice.com
[21]Kueppers F, Black LF. Alpha-1 antitrypsin and its deficiency. Am Rev Respir Dis. 1974;110:176-194.
http://www.ncbi.nlm.nih.gov/pubmed/4212922?tool=bestpractice.com
[22]Tobin MJ, Cook PJ, Hutchison DC. Alpha-1 antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in subjects homozygous for Pi-type-Z. A survey by the British Thoracic Association. Br J Dis Chest. 1983;77:14-27.
http://www.ncbi.nlm.nih.gov/pubmed/6602621?tool=bestpractice.com
[23]Seersholm N, Kok-Jensen A, Dirksen A. Decline in FEV1 among patients with severe hereditary alpha-1 antitrypsin deficiency type Pi Z. Am J Respir Crit Care Med. 1995;152:1922-1925.
http://www.ncbi.nlm.nih.gov/pubmed/8520756?tool=bestpractice.com
The mean age at which smokers with AAT deficiency typically present with symptomatic pulmonary disease is 32 to 41 years.[24]Larsson C. Natural history and life expectancy in severe alpha 1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345-351.
http://www.ncbi.nlm.nih.gov/pubmed/309708?tool=bestpractice.com
Medical history may include asthma and/or granulomatosis with polyangiitis (an infrequent complication of AAT deficiency), and family history may reveal the presence of AAT deficiency in relatives.
Examination findings
General inspection may reveal jaundice, scleral icterus, and/or asterixis if liver disease is present. Abdominal examination may reveal hepatomegaly and/or ascites.
Respiratory examination may reveal wheeze and/or chest hyperinflation if pulmonary disease is present.
Serum AAT measurements
Serum AAT levels should be quantified in individuals with possible AAT deficiency.[1]European Association for the Study of the Liver. EASL Clinical Practice Guidelines on genetic cholestatic liver diseases. J Hepatol. 2024 Aug;81(2):303-25.
https://www.journal-of-hepatology.eu/article/S0168-8278(24)00274-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38851996?tool=bestpractice.com
[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
[53]Guillaud O, Dumortier J, Couchonnal-Bedoya E, et al. Wilson disease and alpha1-antitrypsin deficiency: a review of non-invasive diagnostic tests. Diagnostics (Basel). 2023 Jan 10;13(2):256.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9857715
http://www.ncbi.nlm.nih.gov/pubmed/36673066?tool=bestpractice.com
However, AAT is an acute phase reactant, meaning that normal serum AAT levels can be misleading, especially in the setting of inflammatory processes.[1]European Association for the Study of the Liver. EASL Clinical Practice Guidelines on genetic cholestatic liver diseases. J Hepatol. 2024 Aug;81(2):303-25.
https://www.journal-of-hepatology.eu/article/S0168-8278(24)00274-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38851996?tool=bestpractice.com
[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
Disease states may still be represented by borderline or even normal AAT levels, meaning such results warrant continued suspicion. Serum AAT measurement alone is not recommended for family testing after identification of a proband because it does not fully characterise the risk of disease from AAT deficiency.[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
Some guidelines suggest genotyping for the S and Z allele is the appropriate first step for diagnostic testing of symptomatic individuals.[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
Quantitative testing and protective threshold
Low to normal levels of AAT (<35 micromol/L) should increase suspicion and prompt further testing. Commercially available quantitative testing utilises radial immunodiffusion and nephelometry methods. Exact threshold values vary depending on testing method and regional guidance; appropriate regional guidelines should be consulted for interpretation of serum AAT levels.[6]American Thoracic Society/European Respiratory Society Statement. Standards for the diagnosis and management of individuals with alpha 1-antitrypsin deficiency. Am J Respir Crit Care Med. 2003 Oct 1;168(7):818-900.
https://www.atsjournals.org/doi/full/10.1164/rccm.168.7.818
http://www.ncbi.nlm.nih.gov/pubmed/14522813?tool=bestpractice.com
[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
[9]Lopes AP, Mineiro MA, Costa F, et al. Portuguese consensus document for the management of alpha-1-antitrypsin deficiency. Pulmonology. 2018 Dec;24 Suppl 1:1-21.
https://www.doi.org/10.1016/j.pulmoe.2018.09.004
http://www.ncbi.nlm.nih.gov/pubmed/30473034?tool=bestpractice.com
[10]Dummer J, Dobler CC, Holmes M, et al. Diagnosis and treatment of lung disease associated with alpha one-antitrypsin deficiency: a position statement from the Thoracic Society of Australia and New Zealand. Respirology. 2020 Mar;25(3):321-35.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7078913
http://www.ncbi.nlm.nih.gov/pubmed/32030868?tool=bestpractice.com
Serum AAT level measured using the purified standard developed by the US National Institutes of Health (the most common testing method in the US) are typically given in micromol/L, while AAT levels measured using commercial standards are typically given in mg/dL to differentiate them.[6]American Thoracic Society/European Respiratory Society Statement. Standards for the diagnosis and management of individuals with alpha 1-antitrypsin deficiency. Am J Respir Crit Care Med. 2003 Oct 1;168(7):818-900.
https://www.atsjournals.org/doi/full/10.1164/rccm.168.7.818
http://www.ncbi.nlm.nih.gov/pubmed/14522813?tool=bestpractice.com
Nephelometry values less than 20 micromol/L (83-120 mg/dL) are considered deficient. Nephelometry levels below 11 micromol/L (50 mg/dL) are considered to confer inadequate protection against inflammatory lung disease; this is referred to as the 'protective threshold'.[7]Turino GM, Barker AF, Brantly ML, et al. Clinical features of individuals with PI*SZ phenotype of alpha-1 antitrypsin deficiency: alpha 1-antitrypsin deficiency registry study group. Am J Respir Crit Care Med. 1996;154:1718-1725.
http://www.ncbi.nlm.nih.gov/pubmed/8970361?tool=bestpractice.com
Some of the more common phenotypes result in the following serum AAT levels:
PI*MM: 20-48 micromol/L (150-350 mg/dL)
PI*MZ: 17-33 micromol/L (90-210 mg/dL)
PI*SS: 15-33 micromol/L (100-200 mg/dL)
PI*ZZ: 2.5-7.0 micromol/L (20-45 mg/dL).
Those resulting in levels below the protective threshold are more likely to result in pulmonary disease.
Phenotyping (PI-typing)
Phenotyping can be used when a quick decision is needed, whereas genotyping should be used for definitive diagnosis when available.[1]European Association for the Study of the Liver. EASL Clinical Practice Guidelines on genetic cholestatic liver diseases. J Hepatol. 2024 Aug;81(2):303-25.
https://www.journal-of-hepatology.eu/article/S0168-8278(24)00274-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38851996?tool=bestpractice.com
Low-normal plasma AAT measurements may correspond to heterozygous phenotypes that may place the individual and family members at risk for associated disease. Patients, and first-degree relatives of patients, with normal-low but protective AAT levels (12-35 micromol/L) should undergo qualitative testing through phenotyping.
Phenotyping involves the separation of AAT variants using isoelectric focusing, and can confirm the identification of characteristic deficient AAT-variant proteins. Phenotyping can reveal the presence of the actual protein variants, such as the Z protein, M (normal) protein, and S protein, as well as less common variants.[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
[53]Guillaud O, Dumortier J, Couchonnal-Bedoya E, et al. Wilson disease and alpha1-antitrypsin deficiency: a review of non-invasive diagnostic tests. Diagnostics (Basel). 2023 Jan 10;13(2):256.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9857715
http://www.ncbi.nlm.nih.gov/pubmed/36673066?tool=bestpractice.com
Genotyping
Phenotyping can be used when a quick decision is needed, whereas genotyping should be used for definitive diagnosis when available.[1]European Association for the Study of the Liver. EASL Clinical Practice Guidelines on genetic cholestatic liver diseases. J Hepatol. 2024 Aug;81(2):303-25.
https://www.journal-of-hepatology.eu/article/S0168-8278(24)00274-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38851996?tool=bestpractice.com
Genetic testing may be performed when the actual phenotype does not correspond with the phenotype predicted by the serum AAT level.
It will demonstrate the characteristic AAT alleles responsible for the AAT-variant proteins.
For example, when a low-normal AAT level is detected, further testing with phenotyping is performed to determine the actual AAT protein variants in the serum. If only Z protein is detected, this does not correspond to a low-normal serum AAT level. In this case, additional testing may proceed to genotyping to determine the alleles present in the individual.
Polymerase chain reaction (PCR) is typically used for genotyping. Rare alleles (e.g., null or deficient variants other than Z or S) may require whole gene sequencing. Gene sequencing may also be considered if no primers are available for PCR.[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
[10]Dummer J, Dobler CC, Holmes M, et al. Diagnosis and treatment of lung disease associated with alpha one-antitrypsin deficiency: a position statement from the Thoracic Society of Australia and New Zealand. Respirology. 2020 Mar;25(3):321-35.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7078913
http://www.ncbi.nlm.nih.gov/pubmed/32030868?tool=bestpractice.com
Specific tests for respiratory disease
If respiratory disease is present, pulmonary function testing will demonstrate significantly abnormal results including reduced FEV1.
Chest x-ray may reveal large lung volumes and basilar predominant emphysema. [Figure caption and citation for the preceding image starts]: Chest x-ray of AAT deficiency (PA view)From the personal collection of D. Kyle Hogarth, MD, FCCP; used with permission [Citation ends].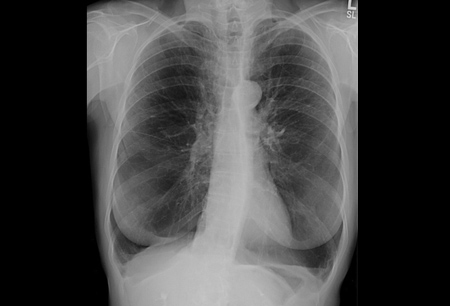 [Figure caption and citation for the preceding image starts]: Chest x-ray of AAT deficiency (lateral view)From the personal collection of D. Kyle Hogarth, MD, FCCP; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Chest x-ray of AAT deficiency (lateral view)From the personal collection of D. Kyle Hogarth, MD, FCCP; used with permission [Citation ends].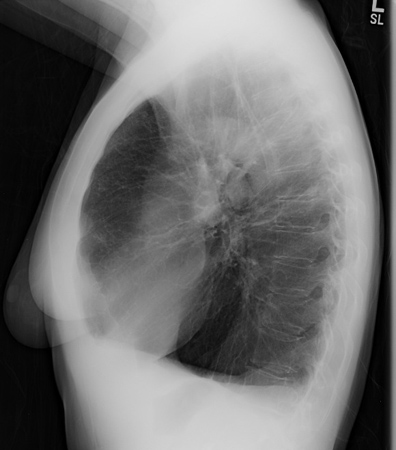
Patients with non-diagnostic results may require a chest computed tomography (CT) scan. CT is more sensitive than chest x-ray or pulmonary function tests for identifying panacinar emphysema and bronchiectasis.[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
[40]Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019 Jan;74(suppl 1):1-69.
https://thorax.bmj.com/content/74/Suppl_1/1.long
http://www.ncbi.nlm.nih.gov/pubmed/30545985?tool=bestpractice.com
However, the absence of emphysematous changes on CT does not rule out AAT deficiency. Panacinar emphysema is predominantly seen in the lower lobes, although upper lobe-only disease has been described. A direct relationship between AAT deficiency and bronchiectasis is less clear as the presence of bronchiectasis on CT may be the result of emphysematous changes.[Figure caption and citation for the preceding image starts]: CT of advanced emphysema in a patient with AAT deficiencyFrom the personal collection of D. Kyle Hogarth, MD, FCCP; used with permission [Citation ends].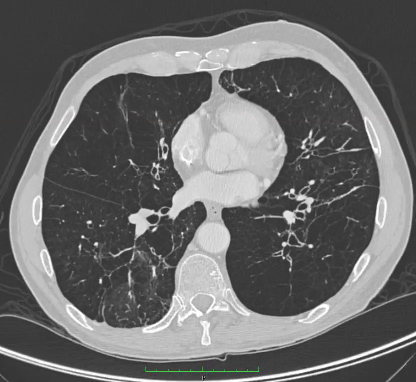
Exercise testing with arterial blood gas analysis in patients with emphysema is also usually abnormal and demonstrates exercise intolerance.[8]Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α<sub>1</sub>-antitrypsin deficiency. Eur Respir J. 2017 Nov 30;50(5):1700610.
https://erj.ersjournals.com/content/50/5/1700610.long
http://www.ncbi.nlm.nih.gov/pubmed/29191952?tool=bestpractice.com
[9]Lopes AP, Mineiro MA, Costa F, et al. Portuguese consensus document for the management of alpha-1-antitrypsin deficiency. Pulmonology. 2018 Dec;24 Suppl 1:1-21.
https://www.doi.org/10.1016/j.pulmoe.2018.09.004
http://www.ncbi.nlm.nih.gov/pubmed/30473034?tool=bestpractice.com
Specific tests for hepatic disease
Guidelines recommend evaluation of liver function with liver function tests (LFTs) for those diagnosed with AAT deficiency, whether symptomatic or asymptomatic for hepatic disease.[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
[53]Guillaud O, Dumortier J, Couchonnal-Bedoya E, et al. Wilson disease and alpha1-antitrypsin deficiency: a review of non-invasive diagnostic tests. Diagnostics (Basel). 2023 Jan 10;13(2):256.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9857715
http://www.ncbi.nlm.nih.gov/pubmed/36673066?tool=bestpractice.com
Alpha-fetoprotein (AFP) levels are also important as part of any liver disease work-up. However, some data suggest that the sensitivity of LFTs, namely alanine aminotransferase (ALT), is only 11.9% in detecting liver disease in AAT deficiency.[54]Clark VC, Dhanasekaran R, Brantly M, et al. Liver test results do not identify liver disease in adults with alpha-1 antitrypsin deficiency. Clin Gastroenterol Hepatol. 2012;10:1278-1283.
http://www.ncbi.nlm.nih.gov/pubmed/22835581?tool=bestpractice.com
Patients with a phenotype associated with liver disease (e.g., PI*ZZ, PI*Mmalton, PI*Siiyama) require liver imaging, and liver ultrasound is recommended annually.[38]Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016 Jun 6;3(3):668-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556762
http://www.ncbi.nlm.nih.gov/pubmed/28848891?tool=bestpractice.com
Liver ultrasound may also be used to monitor for signs of portal hypertension and hepatocellular carcinoma. Abdominal CT and/or magnetic resonance imaging (MRI) can also be helpful for assessing patients for liver morphology, cirrhosis, and portal hypertension, particularly in those with obesity.[53]Guillaud O, Dumortier J, Couchonnal-Bedoya E, et al. Wilson disease and alpha1-antitrypsin deficiency: a review of non-invasive diagnostic tests. Diagnostics (Basel). 2023 Jan 10;13(2):256.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9857715
http://www.ncbi.nlm.nih.gov/pubmed/36673066?tool=bestpractice.com
If hepatocellular carcinoma is present, LFTs may be worsening, and AFP levels may be rising.
As serum liver tests may sometimes yield inconclusive results, the European Association for the Study of the Liver recommends considering liver biopsy in patients with otherwise unexplained, recurrently elevated liver enzymes.[1]European Association for the Study of the Liver. EASL Clinical Practice Guidelines on genetic cholestatic liver diseases. J Hepatol. 2024 Aug;81(2):303-25.
https://www.journal-of-hepatology.eu/article/S0168-8278(24)00274-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38851996?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: Chest x-ray of AAT deficiency (lateral view)From the personal collection of D. Kyle Hogarth, MD, FCCP; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Chest x-ray of AAT deficiency (lateral view)From the personal collection of D. Kyle Hogarth, MD, FCCP; used with permission [Citation ends].
