Approach
Haemoptysis has variously been defined as anything from a small amount of blood-streaked sputum through to massive bleeding with life-threatening consequences due to airway obstruction and haemodynamic instability.[43] Although arbitrary and variable in the literature, the following definitions allow haemoptysis to be clinically characterised by the volume of expectorated blood:
Mild haemoptysis: <30 mL over 24 hours
Frank or moderate haemoptysis: ≥30 mL and <600 mL over 24 hours
Massive haemoptysis: 600 mL or more over 24 hours.
Pathophysiologically, it is intuitive to consider >150 mL haemoptysis as life-threatening, as this volume of blood could flood the conducting airways completely (i.e., fill the anatomic dead space to the level of gas-exchanging lung units). It is important to distinguish between haemoptysis and massive haemoptysis, as the differential diagnosis is narrower for the latter, and therapeutic urgency is greater.
The initial diagnostic assessment should aim to:
Differentiate between haematemesis (the vomiting of blood), pseudohaemoptysis (the coughing of blood from a source other than the lower respiratory tract), and haemoptysis
Identify the site of bleeding
Narrow the differential diagnosis.
History and physical examination
A detailed history and physical examination can help to rule out haematemesis or pseudohaemoptysis, and may provide clues to the site and cause of the haemoptysis.
Pseudohaemoptysis can occur when haematemesis is aspirated into the lungs, or when bleeding from the nasopharynx, sinuses, or oral cavity stimulates a cough reflex.[6] Other rare causes of pseudohaemoptysis include pneumonia due to Serratia marcescens producing a red-pigmented sputum, or sputum pigmentation due to rifampicin use.[44]
Characteristically, haemoptysis tends to be indicated by bright red, frothy sputum that is alkaline and with an oxygen saturation (SaO₂) similar to peripheral arterial saturation. Blood from gastrointestinal sources tends to be darker, may have admixed food particles, is acidic, and has an SaO₂ similar to that found in venous blood.[6][7] The exception is when brisk bleeding in the gastrointestinal tract overcomes the acidic environment of the stomach.
Key features of the history include the following points.
A history of untreated tuberculosis, lung cancer, or extrapulmonary metastatic cancer, and significant weight loss increase the risk for haemoptysis.
A history of smoking or exposure to asbestos or silica confers an increased risk of lung cancer.
A history of chronic mucopurulent sputum production with chronic lung disease is suggestive of bronchiectasis.
A history of exertion, orthopnoea, or paroxysmal nocturnal dyspnoea suggests the presence of congestive heart failure or mitral stenosis.
Dyspnoea and pleuritic chest pain may indicate pulmonary embolism.
Detailed medication history: the use of anticoagulation therapy may indicate coagulopathy. A history of deep venous thrombosis, pulmonary embolism, or hypercoagulable state with inadequate anticoagulation suggests the possibility of pulmonary embolism as the source of haemoptysis.
Travel history to endemic areas: this may point to potential endemic sources of lung infection, such as: histoplasmosis in the Midwest river valleys of the US; coccidioidomycosis in the southwestern US; paragonimiasis in East Asia; or schistosomiasis in the tropics of South America, Africa, and the Far East.
Physical findings are uncommon but may help to establish the cause of haemoptysis.
The presence of ecchymoses and/or petechiae suggests haematological diseases.
The presence of clubbing may be associated with non small-cell bronchogenic carcinoma, bronchiectasis, and chronic lung abscess.
A unilateral wheeze may be heard in cases of bronchial adenoma or endobronchial carcinomas that block the laminar flow of air.
The presence of a diastolic rumble, with an opening snap, loud S1, and loud P2 in the precordial examination, suggest the presence of mitral stenosis.
Other systemic findings such as arthralgias, synovitis, and/or nose deformity are clues to rheumatological causes such as granulomatosis with polyangiitis (formerly Wegener's granulomatosis).
Chest x-ray and chest computed tomography
After a careful history and examination, a chest x-ray (CXR) should be obtained. This has been shown to localise the site of bleeding in 47% of patients.[45] It may also provide clues to any specific entity that may be responsible for haemoptysis, including tuberculosis, malignancy, bronchiectasis, aspergilloma, and lung abscess.
Aspiration of blood into the contralateral lung can cause the CXR to be misleading in determining the side of bleeding.[46] In addition, some causes of haemoptysis may not produce changes on a CXR; these include bronchitis, mild bronchiectasis, small areas of infection, angioma, infarction, aortopulmonary fistula, or an endobronchial lesion that is not large enough to cause bronchial occlusion.[7][47]
Chest computed tomography
If diagnosis remains unclear, chest computed tomography (CT) is indicated. Patients with moderate, or recurrent haemoptysis with high risk for lung cancer (>40 years old and >30 pack-year smoking history) or massive haemoptysis may also benefit from a chest CT scan if it can be done safely.[48][49] The American College of Radiology recommends the use of CT chest with contrast for optimal enhancement of the systemic arterial circulation most commonly implicated in haemoptysis.[50]
A dual source CT scanner enables the rapid acquisition of two x-ray sources at two different energy levels (dual energy mode) simultaneously, allowing for the acquisition of two data sets of diverse information. When coupled with split timing angiography, it enables the simultaneous evaluation of the systemic (bronchial) and pulmonary arteries for localising the source of haemoptysis, including bronchial-pulmonary fistula.[51][52]
If pulmonary embolism is suspected due to acute shortness of breath with or without pleuritic chest pain, a chest CT angiogram or a ventilation/perfusion (V/Q) scan is indicated.[53]
CT chest is also useful to delineate the vascular anatomy prior to therapeutic arterial embolisation.[48]
Bronchoscopy
If the history, exam, and chest imaging do not identify a clear cause for the haemoptysis, bronchoscopy is indicated. Bronchogenic carcinoma is found in 9.6% of patients with haemoptysis with normal CXR.[54]
The flexible bronchoscope is the instrument of choice for evaluating non-massive haemoptysis, as flexible bronchoscopy (FB) can be performed in the outpatient setting, or at the bedside under moderate sedation. FB allows for sub-segmental visualisation of the airways, including the upper lobe orifices.[14] To identify the bleeding site, FB is the most accurate method during active bleeding, with a success rate of 86%, and is widely recognised as the study of choice.[55][56][57] It can also be used as a therapeutic tool to block the bleeding site, and to introduce mechanical or thermal tools to treat it.[29] Although identification of the bleeding bronchopulmonary segment cannot be achieved in every patient, the yield can be increased by examining and performing diagnostic washing in every bronchial orifice. Sometimes, a bleeding tumour can be identified in the sub-segmental bronchus. All abnormalities must be appropriately biopsied, brushed, or lavaged for adequate specimens when possible. The bronchoscopist should pay special attention and document vascular capillaries, bronchial inflammation, and subtle mucosal abnormalities. [Figure caption and citation for the preceding image starts]: Hypervascular endobronchial mucosaFrom the personal collection of Dr Erik Folch [Citation ends].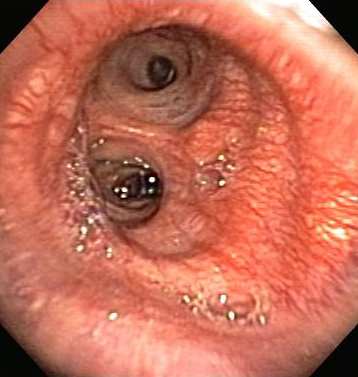
Available studies show a higher success rate when bronchoscopy is carried out early.[55][58][59] Identification of the bleeding site allows the clinician to focus on appropriate treatment. The flexible bronchoscope can be used to evacuate clots from the airway, obtain diagnostic sampling, and deliver local heat- or cold-based therapy.
The use of a rigid rather than a flexible bronchoscope in massive haemoptysis is debated. The choice is mostly driven by the experience of the operator and the clinical scenario. The advantages of the rigid bronchoscope (airway control, larger lumen, the opportunity to use larger instruments, and suction capability) may be offset by the disadvantages (need for an available operating theatre, general anaesthesia, and reduced reach into distal airways).[5][28]
Urgent bronchoscopy in an unstable patient facilitates the introduction of a balloon-tip catheter into the bleeding bronchus to tamponade the haemorrhagic site, thereby protecting the nonbleeding lung from aspiration.[60]
Flexible bronchoscopy and rigid bronchoscopy are considered to be complementary techniques by many experts.[5][14][29][61]
Other imaging techniques
Bronchial arteriography can be used as a diagnostic and therapeutic intervention if available. This technique involves injecting radiocontrast dye into the thoracic aorta to visualise and localise the major systemic arteries to the lung, often guided by localisation of the bleeding source on bronchoscopy. Once the feeding vessels are localised, selective bronchial arteriography can be performed and abnormal vessels identified. Abnormalities may include dilation, tortuosity, hypervascularity, and contrast extravasation. Once the bleeding source is identified, an embolising agent (e.g., polyvinyl alcohol particles, gelfoam, dextran microspheres, or metal coils) can be injected. A post-embolisation arteriogram is performed to ensure complete blockage of the bleeding vessel. The success rates reported with bronchial arteriography range from 70% to 99%, with a recurrence rate of up to 57%.[31]
Neurological complications of paraparesis or paraplegia are potential complications of bronchial arteriography, occurring in 0.6% to 4.4% of procedures, as the anterior spinal artery originates from the bronchial arterial circulation in about 5% of the population.[31][62][63]
Angiography has been compared with flexible bronchoscopy (FB) for the diagnosis of the bleeding site in haemoptysis.[59] FB appeared to have a higher diagnostic yield (particularly when performed early), but angiography was able to identify the bleeding site in 2 out of 8 patients with non-diagnostic bronchoscopies.[59]
CT angiography
Useful for identifying airway and parenchymal disease and vascular anatomy and anomalies. In patients presenting with haemoptysis, it has been shown to provide new diagnostic information in 47%, to clarify abnormalities in 15%, and to localise the site of bleeding in 88%.[46] With the use of iterative model reconstruction and ECG-synchronised prospective-triggered technology in multi-detector-row CT angiography, the bronchial anatomy can be depicted in patients with haemoptysis.[64] In patients with haemoptysis of unknown source and a normal CXR, such CT technology may help identify the possible source of bleeding. The 3-dimensional volume rendering reconstruction allows a virtual trip down the airways and may facilitate the bronchoscopic procedure.[65] The role of this technology in the work-up of patients with haemoptysis remains unclear. From a practical standpoint, CT angiography should be the CT of choice in the initial work-up of patients presenting with haemoptysis, a non-diagnostic CXR, and clinically suspected pulmonary thromboembolic disease. If pulmonary thromboembolism is ruled out, information gleaned from the CT angiogram is usually adequate to map the way for bronchoscopy and for subsequent bronchial artery embolisation, should the latter become necessary. In most cases, identifying a bronchial artery source of haemoptysis is most useful in guiding therapy, which usually means embolisation of the vessel.
Virtual bronchoscopy and CT angiogram, despite the high quality of image renderings, still require conventional bronchial angiography for therapeutic purposes.
Laboratory assessment
The laboratory assessment should be focused towards the suspected diagnosis.
Full blood count may help to identify infection, chronic blood loss, or a haematological disorder (e.g., leukaemia).
Coagulation studies may suggest treatable coagulopathies that facilitate the occurrence of haemoptysis.
Arterial blood gases are indicated, particularly when haemoptysis is severe or there is concern about respiratory failure.
Uraemia should be considered as a factor contributing to haemoptysis due to the adverse effect of uraemia on platelet aggregation.
Urinalysis may help identify a pulmonary-renal syndrome or vasculitis. If there is clinical suspicion for a pulmonary-renal syndrome, an anti-glomerular basement membrane antibody test, anti-neutrophil cytoplasmic antibody test, and/or renal biopsy should be considered.
Sputum and serum studies should be obtained if an infectious cause is suspected. If there is suspicion for granulomatous or cavitary lung infection, sputum collection for acid-fast bacilli and fungal cultures should be obtained. If endemic fungal infection (e.g., histoplasmosis, blastomycosis, coccidioidomycosis) is suspected, fungal serologies should be obtained.[66]
ECG and echocardiogram
If a cardiac cause of haemoptysis is suspected, an ECG and echocardiogram can help to identify the presence of pulmonary hypertension, left ventricular failure, endocarditis, mitral stenosis, or ischaemic heart disease.
How to record an ECG. Demonstrates placement of chest and limb electrodes.
Predictors of mortality
Retrospective data indicate that mechanical ventilation at the time of referral, cancer, aspergillosis, chronic alcoholism, pulmonary artery involvement, and infiltrates involving two quadrants or more on admission are independent predictors of increased mortality among in-hospital patients with haemoptysis.[67]
Use of this content is subject to our disclaimer