Diagnosis of the blast phase of chronic myeloid leukaemia (CML) requires the presence of the Philadelphia chromosome (a genetic mutation that results in the oncogenic BCR::ABL1 fusion gene) in the setting of worsening leukocytosis and increased blast cell count.
Clinical presentations
Anaemia, infections, abnormal/excessive bleeding, bone pain, or constitutional symptoms (night sweats, weight loss, fever) are common presenting complaints of blast-phase CML. Other signs and symptoms include advancing splenomegaly, abdominal pain, cutaneous lesions, early satiety, shoulder pain, anorexia, joint pain, fatigue, petechiae, easy bruising, and visual changes.
Rarely, the disease may present as retinal haemorrhages (visual changes) or hyperviscosity. Clinical features of hyperviscosity include tinnitus, stroke, priapism, confusion, and stupor.[25]Kirwin M, Yee J. Blast crisis. J Educ Teach Emerg Med. 2020 Apr;5(2):S55-S77.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10332564
http://www.ncbi.nlm.nih.gov/pubmed/37465412?tool=bestpractice.com
Initial laboratory testing
Full blood count with differential is the initial laboratory test.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
This should be ordered in patients with fatigue, fever, and/or bleeding disorders, with or without history of CML. Cytopenias and the presence of blast cells serve as clues to diagnosis. These should lead to a peripheral blood smear and bone marrow examination.[Figure caption and citation for the preceding image starts]: Arrow: chronic myeloid leukaemia blast cells with neutrophils in various stages of maturationDr Bruce Villas, Department of Pathology, University of Florida College of Medicine, Jacksonville, FL; used with permission [Citation ends].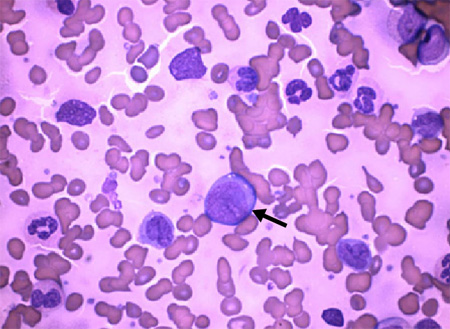
Diagnosis of blast crisis is confirmed by:[1]Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022 Jul;36(7):1703-19.
https://www.nature.com/articles/s41375-022-01613-1
http://www.ncbi.nlm.nih.gov/pubmed/35732831?tool=bestpractice.com
[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[26]Cortes JE, Talpaz M, O'Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006 Mar 15;106(6):1306-15.
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.21756
http://www.ncbi.nlm.nih.gov/pubmed/16463391?tool=bestpractice.com
[27]Arber DA, Orazi A, Hasserjian RP, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022 Sep 15;140(11):1200-28.
https://ashpublications.org/blood/article/140/11/1200/485730/International-Consensus-Classification-of-Myeloid
http://www.ncbi.nlm.nih.gov/pubmed/35767897?tool=bestpractice.com
[28]Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013 Aug 8;122(6):872-84.
https://ashpublications.org/blood/article/122/6/872/32231/European-LeukemiaNet-recommendations-for-the
http://www.ncbi.nlm.nih.gov/pubmed/23803709?tool=bestpractice.com
the percentage of blast cells in the peripheral blood and/or bone marrow (i.e., ≥20% or ≥30% depending on the criteria used), or
the presence of an extramedullary proliferation of blasts.
Subsequent to the introduction of tyrosine kinase inhibitor (TKI) therapy, many clinical trials employ MD Anderson Cancer Center (MDACC) or International Bone Marrow Transplant Registry criteria (blast cells ≥30%).[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
MDACC criteria and International Bone Marrow Transplant Registry criteria are favoured in clinical practice. See Criteria.
Philadelphia chromosome and BCR::ABL1 testing
Cytogenetic testing for the Philadelphia chromosome should be carried out to confirm CML if not previously diagnosed.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[3]Smith G, Apperley J, Milojkovic D, et al; British Society for Haematology. A British Society for Haematology guideline on the diagnosis and management of chronic myeloid leukaemia. Br J Haematol. 2020 Oct;191(2):171-93.
https://onlinelibrary.wiley.com/doi/10.1111/bjh.16971
http://www.ncbi.nlm.nih.gov/pubmed/32734668?tool=bestpractice.com
Several tests are employed during initial evaluation and at follow-up.
Bone marrow aspiration and biopsy: required for cytogenetic analysis (karyotyping) to establish the presence of the Philadelphia chromosome, and to confirm the phase (proportion of blast cells and basophils) of CML.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[29]Cross NCP, Ernst T, Branford S, et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023 Nov;37(11):2150-167.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10624636
http://www.ncbi.nlm.nih.gov/pubmed/37794101?tool=bestpractice.com
Bone marrow cytogenetics can detect additional chromosomal abnormalities (ACAs; also known as clonal cytogenetic evolution) in Ph-positive and Ph-negative cells.[29]Cross NCP, Ernst T, Branford S, et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023 Nov;37(11):2150-167.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10624636
http://www.ncbi.nlm.nih.gov/pubmed/37794101?tool=bestpractice.com
ACAs may be associated with TKI resistance and poor prognosis.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
Molecular analysis using quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) on peripheral blood: should be carried out at initial work-up to establish the presence of quantifiable BCR::ABL1 mRNA transcripts. Laboratories should report qRT-PCR results according to an international scale (IS).[30]Müller MC, Cross NC, Erben P, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009 Nov;23(11):1957-63.
http://www.ncbi.nlm.nih.gov/pubmed/19710700?tool=bestpractice.com
qRT-PCR is highly sensitive and it is the only quantitative method of assessing molecular response to therapy; regular qRT-PCR monitoring is recommended following diagnosis.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[29]Cross NCP, Ernst T, Branford S, et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023 Nov;37(11):2150-167.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10624636
http://www.ncbi.nlm.nih.gov/pubmed/37794101?tool=bestpractice.com
Fluorescence in situ hybridisation (FISH): performed on bone marrow aspirate or peripheral blood to identify BCR::ABL1 rearrangements. Used if bone marrow cytogenetic evaluation is not possible or if results of cytogenetics and qRT-PCR differ. It is sometimes used as an initial screening test or, if qRT-PCR is not available, for disease monitoring. FISH cannot detect ACAs.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[29]Cross NCP, Ernst T, Branford S, et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023 Nov;37(11):2150-167.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10624636
http://www.ncbi.nlm.nih.gov/pubmed/37794101?tool=bestpractice.com
Ancillary tests
Consideration should be given to performing the following tests.
Hepatitis B panel: hepatitis B virus reactivation has been reported in patients receiving TKI therapy. Testing for hepatitis B is recommended before starting TKI treatment so that antiviral prophylaxis can be considered for patients at risk.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[31]Atteya A, Ahmad A, Daghstani D, et al. Evaluation of hepatitis B reactivation among patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer Control. 2020 Jan-Dec;27(1):1073274820976594.
https://journals.sagepub.com/doi/10.1177/1073274820976594
http://www.ncbi.nlm.nih.gov/pubmed/33297765?tool=bestpractice.com
Flow cytometry: may be carried out on bone marrow biopsy (or alternatively peripheral blood) to determine cell lineage (e.g., myeloid, lymphoid, or mixed lineage).[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[32]Hochhaus A, Saussele S, Rosti G, et al; ESMO Guidelines Committee. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28(suppl 4):iv41-51.
https://www.annalsofoncology.org/article/S0923-7534(19)42147-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28881915?tool=bestpractice.com
Mutational analyses: performed to identify mutations associated with TKI resistance. BCR::ABL1 kinase domain mutation analysis (using next generation sequencing) should be carried out for patients who have progressed to blast crisis while receiving TKI therapy, or who have inadequate response to TKI therapy.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[29]Cross NCP, Ernst T, Branford S, et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023 Nov;37(11):2150-167.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10624636
http://www.ncbi.nlm.nih.gov/pubmed/37794101?tool=bestpractice.com
A myeloid mutation panel may be considered in patients with no identified BCR::ABL1 kinase domain mutations to detect low-level BCR::ABL1 kinase domain mutations or BCR::ABL1-independent resistance mutations.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[29]Cross NCP, Ernst T, Branford S, et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023 Nov;37(11):2150-167.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10624636
http://www.ncbi.nlm.nih.gov/pubmed/37794101?tool=bestpractice.com
[32]Hochhaus A, Saussele S, Rosti G, et al; ESMO Guidelines Committee. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28(suppl 4):iv41-51.
https://www.annalsofoncology.org/article/S0923-7534(19)42147-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28881915?tool=bestpractice.com
HLA1 testing: may be carried out early in patients eligible for stem cell transplant to expedite finding a suitable donor.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[32]Hochhaus A, Saussele S, Rosti G, et al; ESMO Guidelines Committee. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28(suppl 4):iv41-51.
https://www.annalsofoncology.org/article/S0923-7534(19)42147-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28881915?tool=bestpractice.com
Lumbar puncture: cerebrospinal fluid should be examined in patients with suspected central nervous system (CNS) involvement. Patients with lymphoid or biphenotypic blast crisis should have lumbar puncture with CNS prophylaxis.[2]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myeloid leukemia [internet publication].
https://www.nccn.org/guidelines/category_1
[3]Smith G, Apperley J, Milojkovic D, et al; British Society for Haematology. A British Society for Haematology guideline on the diagnosis and management of chronic myeloid leukaemia. Br J Haematol. 2020 Oct;191(2):171-93.
https://onlinelibrary.wiley.com/doi/10.1111/bjh.16971
http://www.ncbi.nlm.nih.gov/pubmed/32734668?tool=bestpractice.com
