Criteria
MD Anderson Cancer Center criteria[2][26]
Subsequent to the introduction of tyrosine kinase inhibitor (TKI) therapy, many clinical trials employ MD Anderson Cancer Center (MDACC) criteria for advanced phase CML.[2]
MDACC criteria and International Bone Marrow Transplant Registry criteria are favoured in clinical practice.
One or more of the following:
Blasts ≥30% blasts in the blood, bone marrow, or both
Extramedullary blasts (apart from the spleen).
International Bone Marrow Transplant Registry criteria[2][26]
One or more of the following:
Blasts ≥30% blasts in the blood, bone marrow, or both
Extramedullary blasts (apart from the spleen).
World Health Organization (WHO) criteria[1]
≥20% myeloid blasts in the blood or bone marrow, or
The presence of an extramedullary proliferation of blasts, or
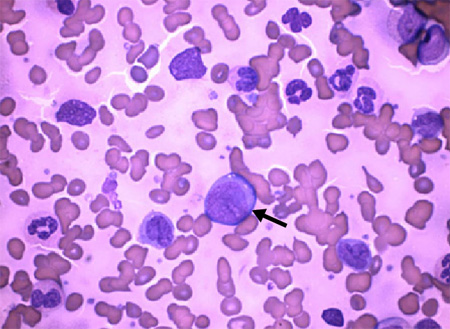
The presence of increased lymphoblasts in peripheral blood or bone marrow.[Figure caption and citation for the preceding image starts]: Arrow: chronic myeloid leukaemia blast cells with neutrophils in various stages of maturationDr Bruce Villas, Department of Pathology, University of Florida College of Medicine, Jacksonville, FL; used with permission [Citation ends].
International consensus classification of myeloid neoplasms and acute leukaemias[27]
≥20% bone marrow or peripheral blood blasts
Extramedullary blast proliferation.
Presence of morphologically apparent lymphoblasts (>5%) warrants consideration of lymphoblastic crisis (and immunophenotypic analysis to confirm lymphoid lineage).
European LeukemiaNet criteria[28]
One or more of the following:
Blasts in blood or marrow ≥30%
Extramedullary blast proliferation (apart from spleen).
Therapeutic response criteria for CML[2]
Complete haematological response:
Complete normalisation of peripheral counts and differential (white blood cell count <10 × 10⁹/L [<10,000/microlitre]; platelet count <450 × 10⁹/L [<450,000/microlitre])
No signs and symptoms of CML, including palpable splenomegaly
No immature myeloid cells (myelocytes, promyelocytes, or blasts) in peripheral blood.
Partial haematological response:
Persistence of few immature myeloid cells, or
Persistence of splenomegaly but >50% improvement from baseline, or
Mild thrombocytosis.
Cytogenetic response is based on the percentage of Philadelphia-positive metaphases:
Complete: 0%
Partial: 1% to 35%
Major: includes complete and partial responses (i.e., ≤35%)
Minor: 36% to 65%
Minimal: 66% to 95%
None: >95%.
Molecular response (MR):
Early: BCR::ABL1 (International Scale [IS]) ≤10% at 3 and 6 months
Major: BCR::ABL1 (IS) ≤0.1% (or ≥3-log reduction in BCR::ABL1 mRNA from the standardised baseline, if quantitative polymerase chain reaction is not available)
Deep: defined as MR4.0: BCR::ABL1 (IS) ≤0.01%; or MR4.5: BCR::ABL1 (IS) ≤0.0032%.
Relapse:
Any sign of loss of haematological response
Any loss of sign of loss of complete cytogenetic response or its molecular response correlate defined as an increase in BCR::ABL1 transcript to >1%
1-log increase in BCR::ABL1 transcript levels with loss of major molecular response.
Use of this content is subject to our disclaimer