Approach
Successful treatment of infectious gangrene requires a combination of surgical debridement, appropriate antibiotics, and intensive supportive care.[17][23][44]
Ischaemic gangrene requires revascularisation for obstruction and thromboembolism, along with treatment of any underlying disease. Measures to prevent superimposed infection must also be performed.
Limb amputation
In cases of severe limb sepsis, amputation is mandated: this is a two-stage procedure starting with guillotine amputation and later, when the infection has cleared, a definitive amputation and wound closure is advisable.[45]
Patients with non-viable extremities (i.e., large amounts of established necrosis, profound anaesthesia, profound paralysis, and inaudible pulse on Doppler), should undergo prompt amputation. The level of amputation is determined by clinical findings and by the viability of tissues at the time of surgery. Every effort should be made to preserve as many joints as possible, in order to decrease the work of ambulating with a prosthesis and to improve the chances for successful rehabilitation.[46]
Necrotising fasciitis
Type I (polymicrobial)
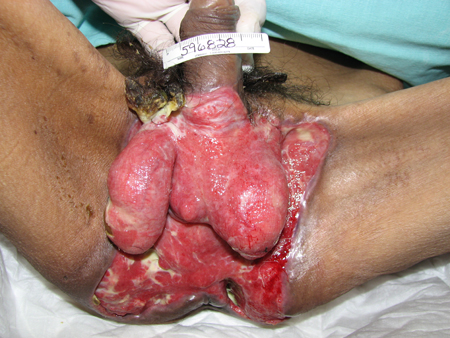
Type I infections are mixed infections with anaerobes. Organisms can include, for example, Bacteroides or Peptostreptococcus with a facultative anaerobe such as the Enterobacteriaceae Escherichia coli, Enterobacter, Klebsiella, or Proteus; or non-group A streptococci. It occurs most frequently after surgical procedures, and in patients with diabetes, alcoholism, immunosuppression, intravenous drug use, or peripheral vascular disease. Fournier's gangrene is a type I necrotising fasciitis of the perineal and genital region, resulting from synergistic polymicrobial infection.[4][Figure caption and citation for the preceding image starts]: Necrotising fasciitis I subtype involving genital and perineal region; image taken after extensive surgical debridementCollection of Jose Contreras-Ruiz (Clinica del Cuidado Integral de Heridas y Estomas) [Citation ends].
Surgical care: wide excision of all necrotic tissue, placement of drains, and appropriate surgical debridement are necessary for both diagnosis and treatment.[44] Following surgical debridement, many surgeons use local irrigation with bacitracin-infused normal saline. Amputation may be required.
Antibiotic therapy: for empirical treatment of type I mixed infections, the Infectious Diseases Society of America (IDSA) recommends agents effective against both aerobes (including MRSA) and anaerobes.[17] Options include vancomycin or linezolid combined with either: piperacillin/tazobactam; a carbapenem; ceftriaxone plus metronidazole; or a fluoroquinolone plus metronidazole. For patients allergic to penicillin, clindamycin or metronidazole with an aminoglycoside or fluoroquinolone may be used. When further information is available and aetiological agent has been determined, antibiotic therapy should be amended to target the specific agent. As there are currently no definitive clinical trials, the IDSA recommends continuing antibiotics until no further surgical debridement is needed, the patient has improved clinically, and fever has been absent for 48 to 72 hours.[17] The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved delafloxacin, a fluoroquinolone antibiotic, for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by designated susceptible bacteria.[47] Delafloxacin reportedly has activity against MRSA and Pseudomonas aeruginosa. In a double-blind phase 2 randomised clinical trial of patients with an ABSSSI, cure rates were significantly greater with delafloxacin than vancomycin, and comparable to those of linezolid.[48]
In November 2018, the EMA completed a review of serious, disabling, and potentially irreversible adverse effects associated with systemic and inhaled fluoroquinolone antibiotics. These adverse effects include tendonitis, tendon rupture, arthralgia, neuropathies, and other musculoskeletal or nervous system effects. As a consequence of this review, the EMA now recommends that fluoroquinolone antibiotics be restricted for use in serious, life-threatening bacterial infections only. Furthermore, it recommends that fluoroquinolones should not be used for mild to moderate infections unless other appropriate antibiotics for the specific infection cannot be used, and should not be used in non-severe, non-bacterial, or self-limiting infections. Patients who are older, have renal impairment, or have had a solid organ transplant, and those being treated with a corticosteroid, are at a higher risk of tendon damage. Co-administration of a fluoroquinolone and a corticosteroid should be avoided.[49] The UK-based Medicines and Healthcare products Regulatory Agency (MHRA) supports these recommendations.[50]
The FDA issued a similar safety communication in 2016, restricting the use of fluoroquinolones in acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections.[51] In addition to these restrictions, the FDA has issued warnings about the increased risk of aortic dissection, significant hypoglycaemia, and mental health adverse effects in patients taking fluoroquinolones.[52][53]
Type II (monomicrobial)
Type II necrotising fasciitis is a monomicrobial infection, a rare form of gangrene most commonly caused by group A (or C or G) streptococci. It usually develops at a site of trauma on an extremity.[1] It can also be caused by MRSA.
Surgical care: immediate surgical exploration is required using longitudinal incisions through the deep fascia and extending beyond the involved gangrenous and undermined areas. Areas of cutaneous necrosis are excised, and non-viable fascia is debrided. Re-exploration is commonly performed within 24 hours.[23][44] Amputation may be required.
Antibiotic therapy: in addition to urgent surgical debridement, penicillin plus clindamycin should be administered to treat group A streptococci and inhibit their ability to synthesise toxins.[17] Clindamycin is used as it has been shown to be superior to penicillin for treatment of experimentally induced necrotising fasciitis or myonecrosis caused by group A streptococci because:[23]
It reduces the in vitro release of streptococcal pyrogenic exotoxin A
It is not affected by inoculum size or stage of growth
It facilitates phagocytosis of Streptococcus pyogenes by inhibiting M-protein synthesis
It suppresses the production of regulatory elements that control cell wall synthesis
It has a long post-antibiotic effect.
If there is any question regarding the aetiological agent (e.g., possibly Staphylococcus aureus rather than a group A Streptococcus), nafcillin should be used.[1]
For patients with penicillin allergy, vancomycin, daptomycin, or linezolid (if co-existent vancomycin allergy) as a monotherapy can be substituted in place of the penicillin-clindamycin or nafcillin-clindamycin combination.[17]
The IDSA recommends a combination of doxycycline plus either ceftriaxone or cefotaxime for necrotising fasciitis due to Vibrio vulnificus, or a combination of doxycycline plus either ceftriaxone or ciprofloxacin for Aeromonas hydrophila.[17]
The addition of intravenous immunoglobulin (IVIG) may also be considered, but efficacy data are conflicting. Some observational studies suggest modest benefit, but a small double-blind placebo-controlled trial (prematurely terminated because of slow patient recruitment) and a large retrospective analysis of the effect of IVIG on patients with debrided necrotising fasciitis (with shock caused by group A Streptococcus or Staphylococcus aureus) found that adjunctive IVIG was not associated with improved survival.[54][55][56][57][58] IDSA guidelines do not include a recommendation regarding the use of IVIG in patients with necrotising fasciitis with streptococcal toxic shock syndrome, citing the need for additional efficacy studies.[17] A 2018 Cochrane review on interventions for necrotising soft tissue infections in adults showed no difference between IVIG (administered over 3 days) and placebo in terms of mortality within 30 days nor in serious adverse events experienced in the ICU (low-certainty evidence).[59] World Society of Emergency Surgery guidelines advocate consideration of IVIG in patients with necrotising fasciitis caused by group A streptococcus and evidence of organ dysfunction (weak recommendation), while recognising that the use of IVIG for treating necrotising soft tissue infections remains controversial.[12]
Hyperbaric oxygen (HBO) therapy can be considered as adjuvant therapy after prompt debridement in patients with necrotising soft tissue infections if no improvement in clinical condition is seen.[12] It is delivered at 100% oxygen at 2 to 3 times atmospheric pressure. While no prospective randomised trials have been published, retrospective analysis has shown an improvement in mortality despite the higher hospitalisation cost and length of stay.[60] There remains a lack of high-quality, valid evidence for the effects of HBO therapy on wound healing. It should not delay prompt surgical exploration and/or empirical antibiotic therapy.
Gas gangrene
Treatment of gas gangrene requires a combination of surgical debridement and antibiotic therapy. Hyperbaric oxygen (HBO) therapy is no longer recommended as it has no proven advantage to the patient and may delay resuscitation and treatment.[17]
Surgical care: aggressive and thorough surgical debridement is mandatory to improve survival, preserve limbs, and prevent complications.[1][6][23] In patients with extremity involvement, fasciotomy may be necessary to treat compartment syndrome, and it should be done immediately after the diagnosis is made. Daily debridement is necessary, and it is extremely important to remove all necrotic and infected tissue. It is also important to consider amputation of the extremity when necessary, as this could be life-saving.
Antibiotic therapy: currently, a combination of penicillin and clindamycin is widely used.[17] Some studies have shown that protein synthesis inhibitors (e.g., clindamycin, chloramphenicol, rifampicin, tetracycline) may be more effective than penicillin because they inhibit the synthesis of clostridial exotoxins and lessen the local and systemic toxic effects of these proteins.[61] For patients allergic to penicillin, a combination of clindamycin and metronidazole is a good choice.
Ischaemic gangrene
For acute limb ischaemia, unless contraindicated, all patients should immediately receive an intravenous heparin bolus, followed by a continuous heparin infusion.[62][63] After the initiation of heparin, treatment then varies depending on the viability of the limb. The following features can help categorise the viability of an acutely ischaemic limb:
Viable: not immediately threatened; no sensory loss or muscle weakness; audible arterial and venous Doppler signals.
Threatened marginally: salvageable if promptly treated; no or minimal (toes) sensory loss; no muscle weakness; often inaudible arterial Doppler signals; audible venous Doppler signals.
Threatened immediately: salvageable with immediate revascularisation; sensory loss of more than toes accompanied by rest pain; mild to moderate muscle weakness; usually inaudible arterial Doppler signals; audible venous Doppler signals.
Irreversible/non-viable: major tissue loss and/or permanent nerve damage; profound anaesthesia; profound paralysis (rigor); inaudible arterial and venous Doppler signals.
Treatment options include surgery, percutaneous transluminal angioplasty (PTA), and thrombolytic therapy.
Surgery
Patients with a threatened but viable extremity (i.e., demonstrating rest pain, sensory loss, or mild muscle weakness, but without substantial areas of necrosis) should undergo urgent surgical revascularisation after heparin anticoagulation. The majority of these patients will have had an embolic event, and irreversible changes can occur within as little as 4 to 6 hours of profound ischaemia. While pharmacological thrombolysis may successfully dissolve an embolus, the time required is usually too long to allow this to be an acceptable alternative to surgery.[63]
In 2010, the bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial result was published based on first treatment received analysis.[64] The outcomes measured were amputation-free survival (AFS) and overall survival (OS). It also compared vein with prosthetic bypass surgery, and transluminal with subintimal balloon angioplasty, and examined outcomes from bypass surgery after failed balloon angioplasty. The author concluded that balloon angioplasty was associated with a significantly higher early failure rate than bypass surgery. Most balloon angioplasty patients ultimately required surgery. Furthermore, bypass surgery outcomes after failed balloon angioplasty are significantly worse than for bypass surgery performed as a first revascularisation attempt. Bypass surgery with vein offers the best long term AFS and OS and, overall, balloon angioplasty appears superior to prosthetic bypass surgery.
Percutaneous transluminal angioplasty (PTA)
In the BASIL trial, 450 patients with severe limb ischaemia due to infra-inguinal disease were randomly assigned to PTA or bypass surgery.[65] At 30 days, there was no difference in mortality between the groups, but surgery was associated with a significantly higher rate of morbidity (57% versus 41%). On intention-to-treat analysis, there was no difference in the primary end point (survival without amputation) at 1 year and 3 years, and surgery was associated with a significantly lower rate of reintervention (18% versus 26%). Based on these findings, the authors recommend that PTA should be offered first to patients with significant comorbidities who are not expected to live more than 2 years. For patients expected to live longer than 2 years, the benefits of bypass surgery could outweigh the short-term increase in morbidity.
Thrombolytic therapy
In highly selected patients, catheter-based intra-arterial thrombolytic therapy may be an alternative to surgery or percutaneous intervention in the management of critical limb ischaemia. The main indication is acute limb ischaemia of less than 14 days' duration in patients with a viable extremity.[46]
Phlegmasia cerulea dolens, a rare condition in which there is total or near-total obstruction of venous drainage from a limb, may be treated by intravenous thrombolytic therapy to help prevent the onset and progression of venous gangrene.[66]
Intensive supportive care
In patients with infective types of gangrene, intractable hypotension and diffuse capillary leak are frequent, and massive amounts of intravenous fluids (10-20 L per day) are often required. In some patients, blood pressure improves with intravenous fluid alone. Pressors may be useful, but there is little information from controlled clinical or experimental studies in this specific condition. Although potent vasoconstrictors such as adrenaline (epinephrine) may improve blood pressure, symmetric gangrene may ensue, partly as a result of the drug and partly as a result of poor perfusion caused by the bacteria, toxins, and endogenous mediators.[17][23]
Use of this content is subject to our disclaimer