Gene-editing therapy, exagamglogene autotemcel, available through managed access agreement in National Health Service (NHS) England
Exagamglogene autotemcel, a gene-editing therapy, has been approved by the National Institute for Health and Care Excellence (NICE) for use in the NHS in England for patients with severe sickle cell anaemia.
Patients aged 12 years and older with βS/βS, βS/β+ or βS/β0 genotype will be eligible for exagamglogene autotemcel, if they:
have recurrent vaso-occlusive crises (at least 2 during the previous 2 years), and
are suitable for haematopoietic stem cell transplant, but a human leukocyte antigen-matched donor is not available.
Exagamglogene autotemcel is a one-time gene therapy that uses CRISPR gene editing technology to alter the patient's own haematopoietic stem cells to produce higher levels of fetal haemoglobin in red blood cells.
The decision to approve exagamglogene autotemcel is based on clinical data from a multiphase, single-arm, open-label trial. In phase 3 of the trial, 29 of 30 patients (97%) who received exagamglogene autotemcel (and had sufficient follow-up to be evaluated) were free from vaso-occlusive crises for at least 12 consecutive months (mean duration of freedom from vaso-occlusive crises: 22.4 months).[102]
Final draft guidance and the managed access agreement are available from the UK NICE.[103]
The managed access agreement is a time-limited agreement that sets out the conditions under which people will be able to have NHS-funded treatment.[104]
Further data will be collected to address the uncertainties in the clinical or cost-effectiveness data.
Voxelotor withdrawn globally due to safety concerns
Voxelotor, indicated for the treatment of sickle cell disease, has been voluntarily withdrawn from the worldwide market by the manufacturer due to serious safety concerns.[93] This includes discontinuing all ongoing clinical trials, as well as compassionate use and expanded access programmes.
Healthcare professionals are advised to:
stop prescribing voxelotor for new patients with sickle cell disease
contact patients already taking voxelotor to advise on stopping treatment and discuss alternative options; patients must talk to their doctor before stopping voxelotor
monitor patients for adverse events after treatment with voxelotor is stopped, and ensure patients are followed-up appropriately
report adverse events to the relevant regulatory body.
The decision to withdraw voxelotor is based on clinical data that indicates the overall benefit of voxelotor no longer outweighs the risk in patients with sickle cell disease.
Higher rates of vaso-occlusive crises and deaths have been reported in patients taking voxelotor compared with placebo in clinical trials. Real-world registry studies also recorded higher rates of vaso-occlusive crisis in patients with sickle cell disease taking voxelotor.
The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) are currently conducting safety reviews of all the available data.[76][77]
Summary
Definition
History and exam
Key diagnostic factors
- parent(s) diagnosed with sickle cell anaemia, other sickle cell disease, or sickle cell trait
- persistent pain in skeleton, chest, and/or abdomen
- dactylitis
Other diagnostic factors
- high temperature
- pneumonia-like syndrome
- bone pain
- visual floaters
- tachypnoea
- failure to thrive
- pallor
- jaundice
- tachycardia
- lethargy
- maxillary hypertrophy with overbite
- protuberant abdomen, often with umbilical hernia
- cardiac systolic flow murmur
- shock
Diagnostic investigations
1st investigations to order
- DNA-based assays
- haemoglobin isoelectric focusing (Hb IEF)
- cellulose acetate electrophoresis
- high-performance liquid chromatography (HPLC)
- haemoglobin solubility testing
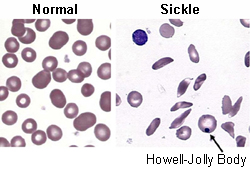
- peripheral blood smear
- FBC and reticulocyte count
- iron studies
Investigations to consider
- pulse oximetry
- plain x-rays of long bones
- bacterial cultures
- chest x-ray
Treatment algorithm
Contributors
Authors
Sophie Lanzkron, MD, MHS
Director
Sickle Cell Center for Adults
Associate Professor of Medicine and Oncology
Johns Hopkins Medicine
Baltimore
MD
Disclosures
SL declares consultancy (Bluebird bio, Novo Nordisk, Pfizer, Novartis, Magenta); honoraria (Novartis); research funding (Imara, Novartis, GBT, Takeda, CSL-Behring, HRSA, PCORI, MD CHRC); stocks (Pfizer, Teva). SL is on the executive board of the National Alliance for Sickle Cell Centers (uncompensated).
Acknowledgements
Dr Sophie Lanzkron would like to gratefully acknowledge Dr Channing Paller, a previous contributor to this topic.
Disclosures
CP declares that she has no competing interests.
Peer reviewers
James Bradner, MD
Instructor in Medicine
Division of Hematologic Neoplasia
Dana-Farber Cancer Institute
Boston
MA
Disclosures
JB declares that he has no competing interests.
Adrian Stephens, MB BS, MD, FRCPath
Consultant Haematologist
University College London Hospitals
London
UK
Disclosures
AS declares that he has no competing interests.
Use of this content is subject to our disclaimer