Anemia should be considered in patients presenting with fatigue, low energy level, pallor, dyspnea on exertion, or pica, as well as in children with growth impairment.
Universal risk factors include pregnancy, vegetarian/vegan diet, menorrhagia, hookworm infection, chronic kidney disease, chronic heart failure, celiac disease, gastrectomy/achlorhydria, and nonsteroidal anti-inflammatory drug use. Preterm or low birth weight and infant feeding with cows’ milk are risk factors in children.
Patients undergoing surgery who have suspected moderate or severe blood loss (>500 mL), and patients with anemia identified preoperatively, should be investigated for anemia.[69]Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017 Feb;72(2):233-47.
https://onlinelibrary.wiley.com/doi/full/10.1111/anae.13773
http://www.ncbi.nlm.nih.gov/pubmed/27996086?tool=bestpractice.com
Clinical evaluation
Key factors in the history specific to IDA include unusual cravings for ice or nonfood items (i.e., pica) and restless legs syndrome.
Physical exam findings include glossitis, angular stomatitis, and nail changes (e.g., thinning, flattening, spooning). Nonspecific findings on history and physical exam include dyspnea, fatigue, pallor, dysphagia, exercise intolerance, impaired muscular performance, growth impairment, dyspepsia, hair loss, and cognitive or behavioral issues.[Figure caption and citation for the preceding image starts]: KoilonychiaReproduced with permission from Bickle Ian. Clinical exam skills: Hand signs BMJ 2004;329:0411402 [Citation ends].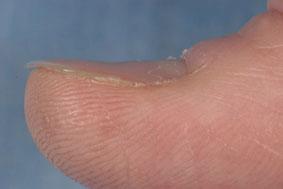
Initial laboratory evaluation with complete blood count and peripheral smear
Initial laboratory testing should include a complete blood count (including hemoglobin and hematocrit, platelet count, mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], mean corpuscular hemoglobin concentration [MCHC], red cell distribution width) with peripheral smear, reticulocyte count, and an iron profile.
The blood count and smear will show a microcytic (low MCV), hypochromic (increased central pallor) anemia.
The World Health Organization defines anemia as: hemoglobin <13 g/dL in men aged ≥15 years; <12 g/dL in nonpregnant women aged ≥15 years; and <11 g/dL in pregnant women.[Figure caption and citation for the preceding image starts]: Peripheral blood smear demonstrating some changes often seen with iron deficiency anemia. Note that many of the red cells are microcytic (compare size of red cell with the lymphocyte nucleus) and hypochromic (wide central pallor). There are some pencil formsFrom personal collection of Dr Rebecca Fischer Connor; used with permission [Citation ends]. A microcytic hypochromic anemia can also be seen in thalassemia and other causes of anemia; therefore, an iron profile evaluation is required to identify iron deficiency as the cause of anemia.
A microcytic hypochromic anemia can also be seen in thalassemia and other causes of anemia; therefore, an iron profile evaluation is required to identify iron deficiency as the cause of anemia.
A reticulocyte count is essential to the workup of all anemias. It determines the number of young red cells being produced and released by the bone marrow. Reticulocyte count is low in IDA.
Iron profile evaluation
The following iron profile is consistent with IDA:[11]Beutler E. Disorders of iron metabolism. In: Lichtman MA, Beutler E, Kipps TJ, et al. Williams hematology. 7th ed. New York: McGraw-Hill Medical; 2006: 511-59,803-22.[70]Daru J, Allotey J, Peña-Rosas JP, et al. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med. 2017 Jun;27(3):167-74.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5763396
http://www.ncbi.nlm.nih.gov/pubmed/28425182?tool=bestpractice.com
[71]World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. 2020 [internet publication].
https://apps.who.int/iris/handle/10665/331505
[72]Fletcher A, Forbes A, Svenson N, et al. Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children. Br J Haematol. 2022 Feb;196(3):523-9.
https://onlinelibrary.wiley.com/doi/10.1111/bjh.17900
http://www.ncbi.nlm.nih.gov/pubmed/34693519?tool=bestpractice.com
Low serum iron
Increased total iron-binding capacity
Transferrin saturation less than 16%
Low serum ferritin (less than 12 nanograms/mL is generally considered diagnostic of IDA, but thresholds vary between guidelines).
Patients with this iron profile do not require further iron tests.
Comments on laboratory evaluation
Many of these tests can be affected by other disorders. For example, ferritin, an acute phase reactant, can be increased in patients with infection or chronic disease (e.g., cancer, autoimmune disorders), which may complicate diagnosis.[72]Fletcher A, Forbes A, Svenson N, et al. Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children. Br J Haematol. 2022 Feb;196(3):523-9.
https://onlinelibrary.wiley.com/doi/10.1111/bjh.17900
http://www.ncbi.nlm.nih.gov/pubmed/34693519?tool=bestpractice.com
The American Gastroenterological Association (AGA) recommends a ferritin cutoff of 45 ng/mL (rather than 15 ng/mL) when ferritin is used to diagnose iron deficiency.[73]Ko CW, Siddique SM, Patel A, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. 2020 Sep;159(3):1085-1094.
https://www.doi.org/10.1053/j.gastro.2020.06.046
http://www.ncbi.nlm.nih.gov/pubmed/32810434?tool=bestpractice.com
The increased ferritin threshold may help to identify patients with IDA in whom inflammation due to rheumatologic disease, chronic infections, or malignancy gives rise to increased ferritin levels (>15 ng/mL). The AGA Technical Review Panel concluded that the trade-off between higher sensitivity and lower specificity using the higher threshold of 45 ng/mL provides an acceptable balance of benefits (fewer missed diagnoses) compared with potential harms (additional diagnostic evaluations).[73]Ko CW, Siddique SM, Patel A, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. 2020 Sep;159(3):1085-1094.
https://www.doi.org/10.1053/j.gastro.2020.06.046
http://www.ncbi.nlm.nih.gov/pubmed/32810434?tool=bestpractice.com
The transferrin receptor-ferritin index can also help diagnose IDA in patients with infection or chronic disease.[74]Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009 Sep;18(3):345-52.
http://www.ncbi.nlm.nih.gov/pubmed/19795030?tool=bestpractice.com
[75]Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997 Feb 1;89(3):1052-7.
http://bloodjournal.hematologylibrary.org/content/89/3/1052.full
http://www.ncbi.nlm.nih.gov/pubmed/9028338?tool=bestpractice.com
Transferrin receptor is increased in iron deficiency and has a high sensitivity and specificity for IDA.[74]Koulaouzidis A, Said E, Cottier R, et al. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009 Sep;18(3):345-52.
http://www.ncbi.nlm.nih.gov/pubmed/19795030?tool=bestpractice.com
[75]Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997 Feb 1;89(3):1052-7.
http://bloodjournal.hematologylibrary.org/content/89/3/1052.full
http://www.ncbi.nlm.nih.gov/pubmed/9028338?tool=bestpractice.com
Bone marrow biopsy is the most sensitive and specific test for IDA, but it is not necessary in most cases and is not completely free of error.[4]Andrews, NC. Iron deficiency and related disorders. In: Lee GR, Foerster J, Lukens J, et al., eds. Wintrobe's clinical hematology. Baltimore, MD: Lippincott, Williams & Wilkins; 1999:979-1010.[11]Beutler E. Disorders of iron metabolism. In: Lichtman MA, Beutler E, Kipps TJ, et al. Williams hematology. 7th ed. New York: McGraw-Hill Medical; 2006: 511-59,803-22. It is usually reserved for patients with unclear serum studies in order to differentiate IDA from anemia of chronic disease.
Follow-up clinical evaluation
The underlying cause of IDA must always be evaluated. Celiac serology, urinalysis, and testing for Helicobacter pylori is recommended for all patients.
If celiac serology is positive or celiac disease is suspected, it should be confirmed with small-bowel biopsy.[76]Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;70(11):2030-51.
https://gut.bmj.com/content/early/2021/09/15/gutjnl-2021-325210.long
http://www.ncbi.nlm.nih.gov/pubmed/34497146?tool=bestpractice.com
Urinalysis is done routinely in all patients to evaluate blood loss from the renal tract.
Test for H pylori using the urea breath test or the fecal antigen test, which are both highly sensitive and specific assays for active H pylori infection.[77]Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017 Feb;112(2):212-39.
https://journals.lww.com/ajg/fulltext/2017/02000/acg_clinical_guideline__treatment_of_helicobacter.12.aspx
http://www.ncbi.nlm.nih.gov/pubmed/28071659?tool=bestpractice.com
Do not request serologic (antibody) testing to detect an active H pylori infection.[78]American Society for Clinical Pathology. Thirty five things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2021 [internet publication].
https://web.archive.org/web/20230316185857/https://www.choosingwisely.org/societies/american-society-for-clinical-pathology
Serology (antibody) testing gives less accurate results, and is unable to distinguish between active and historical infection.[77]Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017 Feb;112(2):212-39.
https://journals.lww.com/ajg/fulltext/2017/02000/acg_clinical_guideline__treatment_of_helicobacter.12.aspx
http://www.ncbi.nlm.nih.gov/pubmed/28071659?tool=bestpractice.com
[79]Chey W, Howden C, Moss S, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2024;119(9):1730-53.
https://journals.lww.com/ajg/fulltext/2024/09000/acg_clinical_guideline__treatment_of_helicobacter.13.aspx?context=featuredarticles&collectionid=5
Gastrointestinal tract investigations
Most patients with IDA and no obvious cause will require gastrointestinal (GI) tract investigations.[76]Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;70(11):2030-51.
https://gut.bmj.com/content/early/2021/09/15/gutjnl-2021-325210.long
http://www.ncbi.nlm.nih.gov/pubmed/34497146?tool=bestpractice.com
Exceptions may include:
menstruating women
patients with a history of obvious blood loss from another system
patients with hypochromic, microcytic anemia whose ethnicity increases the risk of thalassemia, and in whom hemoglobin electrophoresis demonstrates thalassemia.
Upper and lower GI endoscopy are recommended first-line investigations for men and postmenopausal women with newly diagnosed IDA.[73]Ko CW, Siddique SM, Patel A, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. 2020 Sep;159(3):1085-1094.
https://www.doi.org/10.1053/j.gastro.2020.06.046
http://www.ncbi.nlm.nih.gov/pubmed/32810434?tool=bestpractice.com
[76]Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;70(11):2030-51.
https://gut.bmj.com/content/early/2021/09/15/gutjnl-2021-325210.long
http://www.ncbi.nlm.nih.gov/pubmed/34497146?tool=bestpractice.com
If the patient has any upper or lower GI symptoms, the symptomatic part of the GI tract should be investigated first.
If the patient is asymptomatic, both upper and lower GI endoscopy are indicated.
A rectal exam is important and can be done at the same time as lower GI endoscopy. The presence of hemorrhoids as an overt site of bleeding does not preclude further GI exam, which should be performed to look for other proximal lesions (e.g., a neoplasm) in the GI tract.[80]Peytremann-Bridevaux I, Arditi C, Froehlich F, et al; EPAGE II Study Group. Appropriateness of colonoscopy in Europe (EPAGE II). Iron-deficiency anemia and hematochezia. Endoscopy. 2009 Mar;41(3):227-33.
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0028-1119644
http://www.ncbi.nlm.nih.gov/pubmed/19280534?tool=bestpractice.com
Computed tomography colonography can be done if colonoscopy is not available or suitable for the patient.[76]Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;70(11):2030-51.
https://gut.bmj.com/content/early/2021/09/15/gutjnl-2021-325210.long
http://www.ncbi.nlm.nih.gov/pubmed/34497146?tool=bestpractice.com
Small-bowel biopsy is advised during upper GI endoscopy (if done), regardless of celiac serology result. However, it may not be necessary in all patients. For example, if a large bleeding source on colonoscopy is found, then small-bowel biopsy is likely to be of less value. Its use should be determined by the gastroenterologist.
Serologic testing should be carried out if autoimmune gastritis is suspected. Patients with positive antiparietal cell or intrinsic factor antibodies should be referred for endoscopic evaluation.[81]Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014 Jan 16;123(3):326-33.
http://www.bloodjournal.org/content/123/3/326.long?sso-checked=true
http://www.ncbi.nlm.nih.gov/pubmed/24215034?tool=bestpractice.com
Fecal occult blood tests are generally not useful in evaluation of IDA.[76]Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;70(11):2030-51.
https://gut.bmj.com/content/early/2021/09/15/gutjnl-2021-325210.long
http://www.ncbi.nlm.nih.gov/pubmed/34497146?tool=bestpractice.com
If a patient has already been shown to have iron deficiency, a site for potential bleeding must be sought through endoscopy. A fecal occult blood test may; however, be useful in frail patients to screen for GI bleeding to avoid unnecessary invasive testing.[82]Banerjee AK, Celentano V, Khan J, et al. Practical gastrointestinal investigation of iron deficiency anaemia. Expert Rev Gastroenterol Hepatol. 2018 Mar;12(3):249-56.
http://www.ncbi.nlm.nih.gov/pubmed/29129158?tool=bestpractice.com
IDA in premenopausal women is not investigated further with GI endoscopy unless the patient is >50 years old; is not menstruating (e.g., following hysterectomy); has GI symptoms or a strong family history of colorectal cancer; or has recurrent or persistent IDA.[76]Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;70(11):2030-51.
https://gut.bmj.com/content/early/2021/09/15/gutjnl-2021-325210.long
http://www.ncbi.nlm.nih.gov/pubmed/34497146?tool=bestpractice.com
Referral to a gynecologist may be required for evaluation of vaginal causes of bleeding.
Emerging tests
Urinary hepcidin is being evaluated as a noninvasive diagnostic tool for IDA in children.[83]Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016 Jun 9;127(23):2809-13.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4956612
http://www.ncbi.nlm.nih.gov/pubmed/27044621?tool=bestpractice.com
[72]Fletcher A, Forbes A, Svenson N, et al. Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children. Br J Haematol. 2022 Feb;196(3):523-9.
https://onlinelibrary.wiley.com/doi/10.1111/bjh.17900
http://www.ncbi.nlm.nih.gov/pubmed/34693519?tool=bestpractice.com
Percentage of hypochromic erythrocytes, such as decreased MCH, is a late finding in IDA, and its manual calculation can be time consuming. In contrast, the reticulocyte hemoglobin content decreases within the first few days of IDA. Studies to determine the role of reticulocyte hemoglobin content as a biomarker of IDA are ongoing.[84]Löfving A, Domellöf M, Hellström-Westas L, et al. Reference intervals for reticulocyte hemoglobin content in healthy infants. Pediatr Res. 2018 Nov;84(5):657-61.
https://www.nature.com/articles/s41390-018-0046-4
http://www.ncbi.nlm.nih.gov/pubmed/30140071?tool=bestpractice.com
[85]Nalado AM, Mahlangu JN, Duarte R, et al. Utility of reticulocyte haemoglobin content and percentage hypochromic red cells as markers of iron deficiency anaemia among black CKD patients in South Africa. PLoS One. 2018 Oct 3;13(10):e0204899.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6169908
http://www.ncbi.nlm.nih.gov/pubmed/30281654?tool=bestpractice.com
Erythrocyte protoporphyrin is the immediate precursor of hemoglobin, and its levels increase when there is insufficient iron available for hemoglobin production.[8]Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998 Apr 3;47(RR-3):1-29.
http://www.ncbi.nlm.nih.gov/pubmed/9563847?tool=bestpractice.com
The role of erythrocyte protoporphyrin as a screening tool for iron deficiency is being examined.[86]Mei Z, Flores-Ayala RC, Grummer-Strawn LM, et al. Is erythrocyte protoporphyrin a better single screening test for iron deficiency compared to hemoglobin or mean cell volume in children and women? Nutrients. 2017 May 31;9(6):557.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5490536
http://www.ncbi.nlm.nih.gov/pubmed/28561801?tool=bestpractice.com

 A microcytic hypochromic anemia can also be seen in thalassemia and other causes of anemia; therefore, an iron profile evaluation is required to identify iron deficiency as the cause of anemia.
A microcytic hypochromic anemia can also be seen in thalassemia and other causes of anemia; therefore, an iron profile evaluation is required to identify iron deficiency as the cause of anemia.