Achlorhydria is usually a silent incidental finding that, on its own, does not require diagnostic workup or therapeutic intervention. It should be suspected in patients with the following:
Signs and symptoms of anemia due to cobalamin (vitamin B12) or iron deficiency
Signs and symptoms of calcium deficiency
Enteric infection
Decreased efficacy and/or drug levels of certain medications such as thyroxine, ketoconazole, itraconazole, atazanavir, cefpodoxime, enoxacin, and dipyridamole
Hypergastrinemia
Endoscopic findings of thinning of the oxyntic mucosa in the fundus and corpus (body) of the stomach with decreased rugae (gastric folds) and increased visualization of submucosal vessels[67]Eshmuratov A, Nah JC, Kim N, et al. The correlation of endoscopic and histological diagnosis of gastric atrophy. Dig Dis Sci. 2010 May;55(5):1364-75.
http://www.ncbi.nlm.nih.gov/pubmed/19629687?tool=bestpractice.com
Gastric neuroendocrine tumor; carcinoid tumors.
All patients with signs or symptoms suggestive of a gastric hypersecretory disorder or endocrine tumor of the pancreas are recommended to have a serum gastrin performed to diagnose Zollinger-Ellison syndrome. In patients with low hemoglobin, achlorhydria should be suspected as acid facilitates nonheme iron absorption, and intrinsic factor (secreted by parietal cells) is necessary for cobalamin absorption.[68]Hutchinson C, Geissler CA, Powell JJ, et al. Proton-pump inhibitors suppress absorption of dietary non-haem iron in hereditary hemochromatosis. Gut. 2007 Sep;56(9):1291-5.
http://www.ncbi.nlm.nih.gov/pubmed/17344278?tool=bestpractice.com
[69]Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J. 2004 Sep;97(9):887-9.
http://www.ncbi.nlm.nih.gov/pubmed/15455980?tool=bestpractice.com
[70]Annibale B, Capurso G, Chistolini A, et al. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001 Oct 15;111(6):439-45.
http://www.ncbi.nlm.nih.gov/pubmed/11690568?tool=bestpractice.com
[71]Hershko C, Skikne B. Pathogenesis and management of iron deficiency anemia: emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis. Semin Hematol. 2009 Oct;46(4):339-50.
http://www.ncbi.nlm.nih.gov/pubmed/19786202?tool=bestpractice.com
Biopsy of corpus and/or fundus of stomach is the diagnostic standard, and it may be performed when a differential diagnosis of achlorhydria is entertained based on history, physical exam, and/or laboratory findings.[5]El-Zimaity H. Gastritis and gastric atrophy. Curr Opin Gastroenterol. 2008 Nov;24(6):682-6.
http://www.ncbi.nlm.nih.gov/pubmed/19122515?tool=bestpractice.com
[6]Sepulveda AR, Patil M. Practical approach to the pathologic diagnosis of gastritis. Arch Pathol Lab Med. 2008 Oct;132(10):1586-93.
http://www.ncbi.nlm.nih.gov/pubmed/18834216?tool=bestpractice.com
[7]Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005 Mar;36(3):228-33.
http://www.ncbi.nlm.nih.gov/pubmed/15791566?tool=bestpractice.com
[9]Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008 Aug;40(8):650-8.
http://www.ncbi.nlm.nih.gov/pubmed/18424244?tool=bestpractice.com
Patients with hypergastrinemia require evaluation of luminal gastric pH via esophagogastroduodenoscopy (EGD or upper endoscopy) to rule out achlorhydria as etiology prior to embarking on a diagnostic search for Zollinger-Ellison syndrome.
Because Helicobacter pylori, an infection considered carcinogenic by the World Health Organization, plays a role in the pathogenesis of most cases of atrophic gastritis, it is reasonable to test for the organism and, if present, eradicate it.[23]Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009 Feb;24(2):193-201.
http://www.ncbi.nlm.nih.gov/pubmed/19215332?tool=bestpractice.com
[72]Gupta S, Li D, El Serag HB, et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology. 2020 Feb;158(3):693-702.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7340330
http://www.ncbi.nlm.nih.gov/pubmed/31816298?tool=bestpractice.com
[73]Malfertheiner P. Helicobacter pylori infection - management from a European perspective. Dig Dis. 2014;32(3):275-80.
http://www.ncbi.nlm.nih.gov/pubmed/24732193?tool=bestpractice.com
Laboratory findings
A number of laboratory investigations are recommended or merit consideration.
Serum gastrin
Hypergastrinemia is a physiologic response to achlorhydria or hypochlorhydria.[Figure caption and citation for the preceding image starts]: Model illustrating physiologic regulation of gastric acid secretion by gastrin, histamine, somatostatin (SST), and luminal acid. Gastrin, released from antral G cells, is the main hormonal stimulant of acid secretion during meal ingestion. Gastrin acts directly on the acid-secreting parietal cells and, more importantly, indirectly by stimulating histamine secretion from enterochromaffin-like (ECL) cells. Histamine diffuses to adjacent parietal cells, where it binds to histamine H2 receptors coupled to stimulation of acid secretion. In the interdigestive phase, somatostatin (SST), released from antral D cells in response to luminal acid, tonically inhibits gastrin secretion from G cells, thereby maintaining acid secretion at an economically low level.From the collection of Professor Mitchell L. Schubert, with the acknowledgment of Mary Beatty-Brooks (medical illustrator) [Citation ends].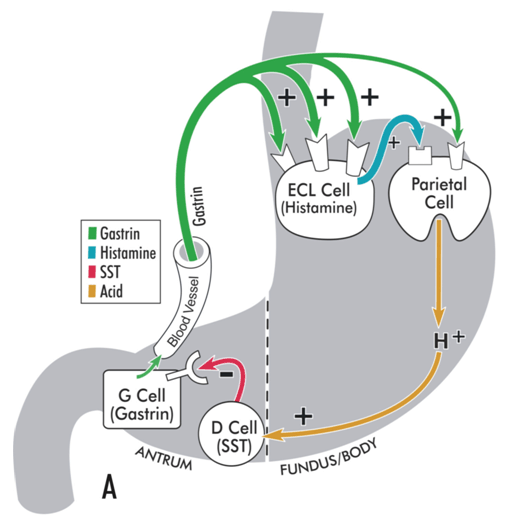 [Figure caption and citation for the preceding image starts]: Model illustrating pathophysiology of achlorhydria and the development of gastric carcinoid tumors. With achlorhydria, the stimulatory effect of luminal acid on SST is lost. Consequently, SST secretion is decreased and its inhibitory restraint on gastrin secretion attenuated (disinhibition), resulting in hypergastrinemia. Gastrin is not only a secretagogue but also a trophic hormone that induces growth of the oxyntic mucosa. If hypergastrinemia is sustained for days, ECL cells will hypertrophy; if sustained for weeks to months, ECL cells become hyperplastic, dysplastic, and, in some patients, become carcinoid tumors.From the collection of Professor Mitchell L. Schubert, with the acknowledgment of Mary Beatty-Brooks (medical illustrator) [Citation ends].
[Figure caption and citation for the preceding image starts]: Model illustrating pathophysiology of achlorhydria and the development of gastric carcinoid tumors. With achlorhydria, the stimulatory effect of luminal acid on SST is lost. Consequently, SST secretion is decreased and its inhibitory restraint on gastrin secretion attenuated (disinhibition), resulting in hypergastrinemia. Gastrin is not only a secretagogue but also a trophic hormone that induces growth of the oxyntic mucosa. If hypergastrinemia is sustained for days, ECL cells will hypertrophy; if sustained for weeks to months, ECL cells become hyperplastic, dysplastic, and, in some patients, become carcinoid tumors.From the collection of Professor Mitchell L. Schubert, with the acknowledgment of Mary Beatty-Brooks (medical illustrator) [Citation ends].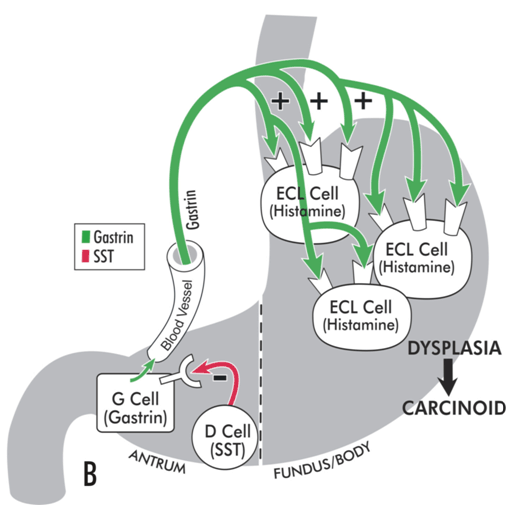
Fasting serum gastrin concentrations in healthy individuals are generally <150 picograms/mL. In patients with achlorhydria, gastrin concentrations are generally >400 picograms/mL and are often >1000 picograms/mL.[74]Klinkenberg-Knol EC, Festen HP, Jansen JB, et al. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med. 1994 Aug 1;121(3):161-7.
http://www.ncbi.nlm.nih.gov/pubmed/8017742?tool=bestpractice.com
[75]McCloy RF, Arnold R, Bardhan KD, et al. Pathophysiological effects of long-term acid suppression in man. Dig Dis Sci. 1995 Feb;40(2 Suppl):96S-120S.
http://www.ncbi.nlm.nih.gov/pubmed/7859587?tool=bestpractice.com
Serum gastrin test should be performed during fasting, and if markedly elevated a gastric pH should be obtained to rule out Zollinger-Ellison syndrome (gastrinoma) as the etiology of the hypergastrinemia.[76]Hung PD, Schubert ML, Mihas AA. Zollinger-Ellison syndrome. Curr Treat Options Gastroenterol. 2003 Apr;6(2):163-70.
http://www.ncbi.nlm.nih.gov/pubmed/12628075?tool=bestpractice.com
Other causes of hypergastrinemia include antisecretory medications (5% of patients on long-term proton-pump inhibitors may have serum gastrin levels exceeding 400 picograms/mL), retained gastric antrum in duodenal limb after antrectomy, renal insufficiency, massive small bowel resection, and gastric outlet obstruction with marked distention.[77]Helgadóttir H, Lund SH, Gizurarson S, et al. Predictors of gastrin elevation following proton pump inhibitor therapy. J Clin Gastroenterol. 2020 Mar;54(3):227-34.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6800823
http://www.ncbi.nlm.nih.gov/pubmed/30994520?tool=bestpractice.com
Intragastric pH
This test may be performed using a pH electrode or by using pH paper. It is more useful in ruling out achlorhydria than in establishing the diagnosis, since reflux of alkaline duodenal contents, in the absence of achlorhydria, can increase the pH of gastric juice to >6.
Intragastric pH testing is often used in hypergastrinemic patients.[76]Hung PD, Schubert ML, Mihas AA. Zollinger-Ellison syndrome. Curr Treat Options Gastroenterol. 2003 Apr;6(2):163-70.
http://www.ncbi.nlm.nih.gov/pubmed/12628075?tool=bestpractice.com
Hemoglobin
Hemoglobin is decreased due to cobalamin (vitamin B12) and/or iron deficiency. As intrinsic factor (IF), which is secreted from parietal cells, is essential for cobalamin absorption, atrophic gastritis is the most common cause of cobalamin deficiency.[15]Howard TA, Misra DN, Grove M, et al. Human gastric intrinsic factor expression is not restricted to parietal cells. J Anat. 1996 Oct;189 (Pt 2):303-13.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1167747/pdf/janat00124-0047.pdf
http://www.ncbi.nlm.nih.gov/pubmed/8886952?tool=bestpractice.com
[78]Scott JM. Folate and vitamin B12. Proc Nutri Soc. 1999 May;58(2):441-8.
http://www.ncbi.nlm.nih.gov/pubmed/10466189?tool=bestpractice.com
[79]Den Elzen WP, Groeneveld Y, De Ruijter W, et al. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol Ther. 2008 Mar 15;27(6):491-7.
http://www.ncbi.nlm.nih.gov/pubmed/18194503?tool=bestpractice.com
About 25% of achlorhydric patients develop iron-deficiency anemia.[68]Hutchinson C, Geissler CA, Powell JJ, et al. Proton-pump inhibitors suppress absorption of dietary non-haem iron in hereditary hemochromatosis. Gut. 2007 Sep;56(9):1291-5.
http://www.ncbi.nlm.nih.gov/pubmed/17344278?tool=bestpractice.com
[69]Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J. 2004 Sep;97(9):887-9.
http://www.ncbi.nlm.nih.gov/pubmed/15455980?tool=bestpractice.com
[70]Annibale B, Capurso G, Chistolini A, et al. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001 Oct 15;111(6):439-45.
http://www.ncbi.nlm.nih.gov/pubmed/11690568?tool=bestpractice.com
[71]Hershko C, Skikne B. Pathogenesis and management of iron deficiency anemia: emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis. Semin Hematol. 2009 Oct;46(4):339-50.
http://www.ncbi.nlm.nih.gov/pubmed/19786202?tool=bestpractice.com
[80]Kowdley KV, Brown KE, Ahn J, et al. ACG clinical guideline: hereditary hemochromatosis. Am J Gastroenterol. 2019 Aug;114(8):1202-18.
https://journals.lww.com/ajg/Fulltext/2019/08000/ACG_Clinical_Guideline__Hereditary_Hemochromatosis.11.aspx
http://www.ncbi.nlm.nih.gov/pubmed/31335359?tool=bestpractice.com
Heme iron (Fe2+), found mainly in the hemoglobin and myoglobin of meat products, represents only 10% to 15% of total dietary iron intake. However, it is particularly well absorbed, and contributes 40% to total iron absorbed. Nonheme iron (Fe3+), found in vegetable products, is soluble only at an acidic pH and precipitates at pH >4. Because gastric acid releases Fe3+ from food and reduces it to ferrous iron (Fe2+), achlorhydria reduces iron absorption.
Pernicious anemia describes cobalamin deficiency that results from impaired secretion of intrinsic factor due to atrophy of the oxyntic mucosa.[81]Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol. 2009 Nov 7;15(41):5121-8.
http://www.wjgnet.com/1007-9327/full/v15/i41/5121.htm
http://www.ncbi.nlm.nih.gov/pubmed/19891010?tool=bestpractice.com
Pernicious anemia is considered an autoimmune disorder due to the frequent presence of gastric autoantibodies directed against IF and parietal cells.
Intrinsic factor antibodies
Intrinsic factor (IF), a glycoprotein secreted by parietal cells and, to a lesser degree, chief cells, is necessary for the absorption of cobalamin (vitamin B12).[15]Howard TA, Misra DN, Grove M, et al. Human gastric intrinsic factor expression is not restricted to parietal cells. J Anat. 1996 Oct;189 (Pt 2):303-13.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1167747/pdf/janat00124-0047.pdf
http://www.ncbi.nlm.nih.gov/pubmed/8886952?tool=bestpractice.com
Because cobalamin body stores are 1000-fold the daily requirement, it takes many years for a patient with gastric atrophy to develop cobalamin deficiency.[78]Scott JM. Folate and vitamin B12. Proc Nutri Soc. 1999 May;58(2):441-8.
http://www.ncbi.nlm.nih.gov/pubmed/10466189?tool=bestpractice.com
[79]Den Elzen WP, Groeneveld Y, De Ruijter W, et al. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol Ther. 2008 Mar 15;27(6):491-7.
http://www.ncbi.nlm.nih.gov/pubmed/18194503?tool=bestpractice.com
Over 70% of patients with gastric atrophy and/or autoimmune gastritis have antibodies directed against the parietal cell hydrogen-potassium-stimulated adenosine triphosphatase (H+/K+ ATPase) and/or IF.[31]Davidson RJ, Atrah HI, Sewell HF. Longitudinal study of circulating gastric antibodies in pernicious anaemia. J Clin Pathol. 1989 Oct;42(10):1092-5.
http://jcp.bmj.com/content/42/10/1092.long
http://www.ncbi.nlm.nih.gov/pubmed/2584410?tool=bestpractice.com
[36]Lewerin C, Jacobsson S, Lindstedt G, et al. Serum biomarkers for atrophic gastritis and antibodies against Helicobacter pylori in the elderly: implications for vitamin B12, folic acid, and iron status and response to oral vitamin therapy. Scand J Gastroenterol. 2008;43(9):1050-6.
http://www.ncbi.nlm.nih.gov/pubmed/18609169?tool=bestpractice.com
[37]D'Elios MM, Bergman MP, Azzurri A, et al. H+,K+-ATPase (proton-pump) is the target autoantigen of TH1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001 Feb;120(2):377-86.
http://www.ncbi.nlm.nih.gov/pubmed/11159878?tool=bestpractice.com
[38]Claeys D, Faller G, Appelmelk BJ, et al. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998 Aug;115(2):340-7.
http://www.ncbi.nlm.nih.gov/pubmed/9679039?tool=bestpractice.com
IF antibodies are >95% specific and 50% to 85% sensitive for pernicious anemia.[78]Scott JM. Folate and vitamin B12. Proc Nutri Soc. 1999 May;58(2):441-8.
http://www.ncbi.nlm.nih.gov/pubmed/10466189?tool=bestpractice.com
[82]Richter C, Tanaka T, Yada RY. Mechanism of activation of the gastric aspartic proteinases: pepsinogen, progastricsin and prochymosin. Biochem J. 1998 Nov 1;335 (Pt 3):481-90.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1219805/pdf/9794784.pdf
http://www.ncbi.nlm.nih.gov/pubmed/9794784?tool=bestpractice.com
[83]Xu D, Fyfe JC. Cubilin expression and posttranslational modification in the canine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2000 Oct;279(4):G748-56.
http://www.ncbi.nlm.nih.gov/pubmed/11005762?tool=bestpractice.com
[84]Moestrup SK. New insights into carrier binding and epithelial uptake of the erythropoietic nutrients cobalamin and folate. Curr Opin Hematol. 2006 May;13(3):119-23.
http://www.ncbi.nlm.nih.gov/pubmed/16567952?tool=bestpractice.com
Parietal cell antibodies
Parietal cell antibodies, directed against the alpha and beta subunits of the parietal cell H+/K+ ATPase, are present in up to 90% of patients with pernicious anemia.
Parietal cell antibodies may be acquired due to molecular mimicry between Helicobacter pylori lipopolysaccharide and H+/K+ ATPase, both of which contain Lewis epitopes.[59]Annibale B, Lahner E, Santucci A, et al. CagA and VacA are immunoblot markers of past Helicobacter pylori infection in atrophic body gastritis. Helicobacter. 2007 Feb;12(1):23-30.
http://www.ncbi.nlm.nih.gov/pubmed/17241297?tool=bestpractice.com
With progression of the gastritis, the incidence of the antibodies may decrease to about 55% to 80%, presumably because of the loss of antigenic drive.[30]Toh BH, Alderuccio F. Pernicious anemia. Autoimmunity. 2004 Jun;37(4):357-61.
http://www.ncbi.nlm.nih.gov/pubmed/15518059?tool=bestpractice.com
[31]Davidson RJ, Atrah HI, Sewell HF. Longitudinal study of circulating gastric antibodies in pernicious anaemia. J Clin Pathol. 1989 Oct;42(10):1092-5.
http://jcp.bmj.com/content/42/10/1092.long
http://www.ncbi.nlm.nih.gov/pubmed/2584410?tool=bestpractice.com
[36]Lewerin C, Jacobsson S, Lindstedt G, et al. Serum biomarkers for atrophic gastritis and antibodies against Helicobacter pylori in the elderly: implications for vitamin B12, folic acid, and iron status and response to oral vitamin therapy. Scand J Gastroenterol. 2008;43(9):1050-6.
http://www.ncbi.nlm.nih.gov/pubmed/18609169?tool=bestpractice.com
[37]D'Elios MM, Bergman MP, Azzurri A, et al. H+,K+-ATPase (proton-pump) is the target autoantigen of TH1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001 Feb;120(2):377-86.
http://www.ncbi.nlm.nih.gov/pubmed/11159878?tool=bestpractice.com
[38]Claeys D, Faller G, Appelmelk BJ, et al. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998 Aug;115(2):340-7.
http://www.ncbi.nlm.nih.gov/pubmed/9679039?tool=bestpractice.com
In one study, combining IF antibody and parietal cell antibody testing yielded a 60% sensitivity for gastric atrophy and 73% sensitivity for pernicious anemia.[52]Liaskos C, Norman GL, Moulas A, et al. Prevalence of gastric parietal cell antibodies and intrinsic factor antibodies in primary biliary cirrhosis. Clin Chim Acta. 2010 Mar;411(5-6):411-5.
http://www.ncbi.nlm.nih.gov/pubmed/20026019?tool=bestpractice.com
Gastric acid secretory test (gastric analysis)
Gastric acid secretory testing is the definitive test for the diagnosis of achlorhydria, but is not widely available or performed.[85]Oh DS, Wang HS, Ohning GV, et al. Validation of a new endoscopic technique to assess acid output in Zollinger-Ellison Syndrome. Clin Gastroenterol Hepatol. 2006 Dec;4(12):1467-73.
http://www.ncbi.nlm.nih.gov/pubmed/17101299?tool=bestpractice.com
[86]Moore EW, Scarlata RW. The determination of gastric acidity by the glass electrode. Gastroenterology. 1965 Aug;49:178-88.
http://www.ncbi.nlm.nih.gov/pubmed/14323728?tool=bestpractice.com
It may be considered (in specialized centers) when the diagnosis remains in doubt after less invasive testing.
The test is performed by placing a nasogastric tube into the most dependent portion of the stomach during fasting and aspirating gastric juice by suction. Proper positioning may be verified fluoroscopically or by recovery of >90 mL after injection of 100 mL water.
The H+ concentration in a sample of gastric juice is determined either by back-titration to pH 7.0 using a base (e.g., sodium hydroxide) or by measuring the pH of the sample with an electrode and converting this to concentration using a table of activity coefficients for H+ in gastric juice. Once the H+ concentration of the sample in mmol per liter is determined, it is multiplied by the volume of the sample in liters to determine the acid output during the collection period.
Basal acid output (BAO) estimates resting acid secretion and is expressed as the sum of the measured acid output, expressed as mmol H+ per hour, for 4 consecutive 15-minute periods. Maximal acid output (MAO) and peak acid output (PAO) estimate the acid secretory response to an exogenous secretagogue. MAO is the sum of acid output of 4 consecutive 15-minute periods, and PAO is calculated by multiplying by 2 the sum of the 2 highest outputs recorded in the four 15-minute test periods.
Biopsy of corpus and/or fundus of stomach
There is poor correlation between the endoscopic visual determination of gastric atrophy and the histologic diagnosis of achlorhydria.[67]Eshmuratov A, Nah JC, Kim N, et al. The correlation of endoscopic and histological diagnosis of gastric atrophy. Dig Dis Sci. 2010 May;55(5):1364-75.
http://www.ncbi.nlm.nih.gov/pubmed/19629687?tool=bestpractice.com
The diagnosis of gastric atrophy with achlorhydria is most often made by the finding of atrophic gastritis on biopsy of the oxyntic mucosa at time of esophagogastroduodenoscopy (EGD or upper endoscopy) together with the finding of a pH >6 on gastric fluid aspirated during EGD and/or hypergastrinemia.
Gastric atrophy is characterized by loss of glands and parietal cells, with a decreased ratio of the area occupied by glands to the total mucosa area.[87]Al-Omari FA, Matalka II, Al-Jarrah MA, et al. An intelligent decision support system for quantitative assessment of gastric atrophy. J Clin Pathol. 2011 Apr;64(4):330-7.
http://www.ncbi.nlm.nih.gov/pubmed/21345875?tool=bestpractice.com
Gastric atrophy is usually associated with gastric intestinal metaplasia (IM), the latter may be spotty and hence missed with limited endoscopic biopsies. Because gastric atrophy and gastric IM usually occur on a background of chronic gastritis, some use the term "atrophic gastritis."[5]El-Zimaity H. Gastritis and gastric atrophy. Curr Opin Gastroenterol. 2008 Nov;24(6):682-6.
http://www.ncbi.nlm.nih.gov/pubmed/19122515?tool=bestpractice.com
[6]Sepulveda AR, Patil M. Practical approach to the pathologic diagnosis of gastritis. Arch Pathol Lab Med. 2008 Oct;132(10):1586-93.
http://www.ncbi.nlm.nih.gov/pubmed/18834216?tool=bestpractice.com
[7]Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005 Mar;36(3):228-33.
http://www.ncbi.nlm.nih.gov/pubmed/15791566?tool=bestpractice.com
[9]Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008 Aug;40(8):650-8.
http://www.ncbi.nlm.nih.gov/pubmed/18424244?tool=bestpractice.com
Autoimmune atrophic gastritis is characterized by lymphocytic infiltration into the epithelium (98%), muscularis mucosa thickening (93%), gland shortening and branching (87%), basal lymphoid aggregates (83%), eosinophil infiltration (46%), and neutrophil infiltration (44%).[88]Bettington M, Brown I. Autoimmune gastritis: novel clues to histological diagnosis. Pathology. 2013 Feb;45(2):145-9.
http://www.ncbi.nlm.nih.gov/pubmed/23277173?tool=bestpractice.com
Post-histologic diagnosis of gastric IM
Multiple biopsies (two from the antrum [at lesser and greater curvature], two from the corpus [at lesser and greater curvature], and one from the incisura) should be obtained.[89]Graham DY, Rugge M, Genta RM. Diagnosis: gastric intestinal metaplasia - what to do next? Curr Opin Gastroenterol. 2019 Nov;35(6):535-43.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6900998
http://www.ncbi.nlm.nih.gov/pubmed/31415250?tool=bestpractice.com
Labeling of the areas biopsied is essential as it helps differentiate normal antral mucosa from pseudopyloric metaplasia of the corpus. Antral and corpus samples, as well as tissue from abnormal mucosa and normal-appearing mucosa, should be placed in separate containers for histopathologic preparation and analysis.[89]Graham DY, Rugge M, Genta RM. Diagnosis: gastric intestinal metaplasia - what to do next? Curr Opin Gastroenterol. 2019 Nov;35(6):535-43.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6900998
http://www.ncbi.nlm.nih.gov/pubmed/31415250?tool=bestpractice.com
Helicobacter pylori infection
H pylori infection is probably the most important contributory factor for the development of chronic atrophic gastritis with achlorhydria, even though most patients harboring the organism are not achlorhydric. Although there are few data to support the premise that eradication of the organism once atrophy and IM develop will either cease or reverse the process or prevent the development of adenocarcinoma, most gastroenterologists specializing in this field would recommend eradication of the organism if it is still present.[72]Gupta S, Li D, El Serag HB, et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology. 2020 Feb;158(3):693-702.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7340330
http://www.ncbi.nlm.nih.gov/pubmed/31816298?tool=bestpractice.com
[90]Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007 Aug;102(8):1808-25.
https://deepblue.lib.umich.edu/handle/2027.42/73792
http://www.ncbi.nlm.nih.gov/pubmed/17608775?tool=bestpractice.com
[91]Malfertheiner P, Megraud F, O'Morain CA, et al; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection - the Maastricht V/Florence Consensus Report. Gut. 2017 Jan;66(1):6-30.
https://gut.bmj.com/content/66/1/6.long
http://www.ncbi.nlm.nih.gov/pubmed/27707777?tool=bestpractice.com
[92]Hwang YJ, Kim N, Lee HS, et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment Pharmacol Ther. 2018 Feb;47(3):380-90.
http://www.ncbi.nlm.nih.gov/pubmed/29193217?tool=bestpractice.com
[93]Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018 Mar 22;378(12):1085-95.
https://www.nejm.org/doi/10.1056/NEJMoa1708423?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0www.ncbi.nlm.nih.gov
http://www.ncbi.nlm.nih.gov/pubmed/29562147?tool=bestpractice.com
[94]Malfertheiner P. Gastric atrophy reversible or irreversible after Helicobacter pylori eradication - an open question. Digestion. 2011;83(4):250-2.
https://www.karger.com/Article/Pdf/321529
http://www.ncbi.nlm.nih.gov/pubmed/21273773?tool=bestpractice.com
The American Gastroenterological Association reports that H pylorieradication among individuals with or without gastric IM is associated with a 32% risk reduction for gastric cancer.[76]Hung PD, Schubert ML, Mihas AA. Zollinger-Ellison syndrome. Curr Treat Options Gastroenterol. 2003 Apr;6(2):163-70.
http://www.ncbi.nlm.nih.gov/pubmed/12628075?tool=bestpractice.com
However, there was a lack of data on the impact of H pylorieradication in those with confirmed gastric IM, and most enrolled patients were from an indigenous Chinese population with an increased risk for gastric cancer.
Diagnostic tests for H pyloriinfection
Diagnostic tests for H pylori infection, each with >90% sensitivity and >90% specificity, include histology with immunohistochemical stain, urea breath test, rapid urease test on biopsy samples, polymerase chain reaction, fluorescence in situ hybridization, and stool antigen test.[90]Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007 Aug;102(8):1808-25.
https://deepblue.lib.umich.edu/handle/2027.42/73792
http://www.ncbi.nlm.nih.gov/pubmed/17608775?tool=bestpractice.com
[95]Elitsur Y, Tolia V, Gilger MA, et al. Urea breath test in children: the United States prospective, multicenter study. Helicobacter. 2009 Apr;14(2):134-40.
http://www.ncbi.nlm.nih.gov/pubmed/19298341?tool=bestpractice.com
[96]Shimoyama T, Oyama T, Matsuzaka M, et al. Comparison of a stool antigen test and serology for the diagnosis of Helicobacter pylori infection in mass survey. Helicobacter. 2009 Apr;14(2):87-90.
http://www.ncbi.nlm.nih.gov/pubmed/19298335?tool=bestpractice.com
[97]Granstrom M, Lehours P, Bengtsson C, et al. Diagnosis of Helicobacter pylori. Helicobacter. 2008 Oct;13(suppl 1):7-12.
http://www.ncbi.nlm.nih.gov/pubmed/18783515?tool=bestpractice.com
[98]Stenstrom B, Mendis A, Marshall B. Helicobacter pylori - the latest in diagnosis and treatment. Aus Fam Physician. 2008 Aug;37(8):608-12.
http://www.ncbi.nlm.nih.gov/pubmed/18704207?tool=bestpractice.com
[99]Calvet X, Ramírez Lázaro MJ, Lehours P, et al. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter. 2013 Sep;18(suppl 1):5-11.
http://onlinelibrary.wiley.com/doi/10.1111/hel.12071/full
http://www.ncbi.nlm.nih.gov/pubmed/24011238?tool=bestpractice.com
Serology is >90% sensitive, but is <80% specific for active infection, since antibodies may remain detectable years after the organism is eradicated.[9]Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008 Aug;40(8):650-8.
http://www.ncbi.nlm.nih.gov/pubmed/18424244?tool=bestpractice.com
[97]Granstrom M, Lehours P, Bengtsson C, et al. Diagnosis of Helicobacter pylori. Helicobacter. 2008 Oct;13(suppl 1):7-12.
http://www.ncbi.nlm.nih.gov/pubmed/18783515?tool=bestpractice.com
[98]Stenstrom B, Mendis A, Marshall B. Helicobacter pylori - the latest in diagnosis and treatment. Aus Fam Physician. 2008 Aug;37(8):608-12.
http://www.ncbi.nlm.nih.gov/pubmed/18704207?tool=bestpractice.com
Consequently, serology should not be used to document eradication of infection. Where available, other diagnostic tests (stool antigen, breath test) should instead be used to test for active infection.[100]American Society for Clinical Pathology. Thirty five things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2021 [internet publication].
https://web.archive.org/web/20230316185857/https://www.choosingwisely.org/societies/american-society-for-clinical-pathology
 [Figure caption and citation for the preceding image starts]: Model illustrating pathophysiology of achlorhydria and the development of gastric carcinoid tumors. With achlorhydria, the stimulatory effect of luminal acid on SST is lost. Consequently, SST secretion is decreased and its inhibitory restraint on gastrin secretion attenuated (disinhibition), resulting in hypergastrinemia. Gastrin is not only a secretagogue but also a trophic hormone that induces growth of the oxyntic mucosa. If hypergastrinemia is sustained for days, ECL cells will hypertrophy; if sustained for weeks to months, ECL cells become hyperplastic, dysplastic, and, in some patients, become carcinoid tumors.From the collection of Professor Mitchell L. Schubert, with the acknowledgment of Mary Beatty-Brooks (medical illustrator) [Citation ends].
[Figure caption and citation for the preceding image starts]: Model illustrating pathophysiology of achlorhydria and the development of gastric carcinoid tumors. With achlorhydria, the stimulatory effect of luminal acid on SST is lost. Consequently, SST secretion is decreased and its inhibitory restraint on gastrin secretion attenuated (disinhibition), resulting in hypergastrinemia. Gastrin is not only a secretagogue but also a trophic hormone that induces growth of the oxyntic mucosa. If hypergastrinemia is sustained for days, ECL cells will hypertrophy; if sustained for weeks to months, ECL cells become hyperplastic, dysplastic, and, in some patients, become carcinoid tumors.From the collection of Professor Mitchell L. Schubert, with the acknowledgment of Mary Beatty-Brooks (medical illustrator) [Citation ends].