Necrotizing fasciitis is a surgical emergency, requiring rapid debridement of the infected subcutaneous tissues, in combination with empiric antibiotic therapy directed broadly at the likely etiologic agents.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
[20]Angoules AG, Kontakis G, Drakoulakis E, et al. Necrotising fasciitis of upper and lower limb: a systematic review. Injury. 2007 Dec;38(suppl 5):S19-26.
http://www.ncbi.nlm.nih.gov/pubmed/18048033?tool=bestpractice.com
[38]Gelbard RB, Ferrada P, Yeh DD, et al. Optimal timing of initial debridement for necrotizing soft tissue infection: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2018 Jul;85(1):208-14.
http://www.ncbi.nlm.nih.gov/pubmed/29485428?tool=bestpractice.com
Tailoring of antimicrobial therapy, as appropriate, is recommended when causative microbial organism(s) are identified via culture.
A combined team approach (surgeon, infectious disease specialist, microbiologist) provides the basis for optimal management.[15]Hua C, Urbina T, Bosc R, et al. Necrotising soft-tissue infections. Lancet Infect Dis. 2023 Mar;23(3):e81-94.
http://www.ncbi.nlm.nih.gov/pubmed/36252579?tool=bestpractice.com
[43]Peetermans M, de Prost N, Eckmann C, et al. Necrotizing skin and soft-tissue infections in the intensive care unit. Clin Microbiol Infect. 2020 Jan;26(1):8-17.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30382-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31284035?tool=bestpractice.com
This topic covers the diagnosis and management of necrotizing fasciitis in adults only.
Initial management
A surgical consultation should be obtained as soon as the diagnosis is suspected.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
[43]Peetermans M, de Prost N, Eckmann C, et al. Necrotizing skin and soft-tissue infections in the intensive care unit. Clin Microbiol Infect. 2020 Jan;26(1):8-17.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30382-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31284035?tool=bestpractice.com
Surgical debridement should be performed as soon as possible, but at least within 12 hours of hospital admission.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[38]Gelbard RB, Ferrada P, Yeh DD, et al. Optimal timing of initial debridement for necrotizing soft tissue infection: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2018 Jul;85(1):208-14.
http://www.ncbi.nlm.nih.gov/pubmed/29485428?tool=bestpractice.com
The infected subcutaneous tissue is devitalized, so expedited surgical removal of infected tissue is critical for successful treatment. Delay in surgical debridement (>12 hours after admission) has been associated with the need for a greater number of subsequent debridements, higher incidence of organ failure, and higher mortality.[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
[38]Gelbard RB, Ferrada P, Yeh DD, et al. Optimal timing of initial debridement for necrotizing soft tissue infection: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2018 Jul;85(1):208-14.
http://www.ncbi.nlm.nih.gov/pubmed/29485428?tool=bestpractice.com
[47]Kobayashi L, Konstantinidis A, Shackelford S, et al. Necrotizing soft tissue infections: delayed surgical treatment is associated with increased number of surgical debridements and morbidity. J Trauma. 2011 Nov;71(5):1400-5.
http://www.ncbi.nlm.nih.gov/pubmed/21768906?tool=bestpractice.com
While surgery is awaited, patients should be monitored for systemic toxicity (e.g., signs of end-organ damage), as well as local signs and symptoms of extension of the area of necrotizing fasciitis.[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
Empiric antibiotic therapy should be started immediately, then tailored based on culture results from subcutaneous tissue or blood, the patient’s clinical condition, and discussion with the multidisciplinary team.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
An early review from the critical care team should be sought; intensive hemodynamic support with intravenous fluids, and possibly vasoactive drugs, will be needed.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
[43]Peetermans M, de Prost N, Eckmann C, et al. Necrotizing skin and soft-tissue infections in the intensive care unit. Clin Microbiol Infect. 2020 Jan;26(1):8-17.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30382-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31284035?tool=bestpractice.com
When debridement is performed, surgical incisions should extend beyond the areas of visible necrosis and the entire necrotic area excised.[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
Surgical specimens including tissue and fluid should be obtained for pathology and microbiological culture.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
Further surgical evaluation and debridement is necessary in most cases, and several procedures may be required to ensure that all necrotic tissue is removed. Data to guide optimal timing for surgical re-exploration is lacking; a reasonable approach may be serial debridement every 12 to 24 hours until minimal or no remaining necrotic tissue is encountered.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
Streptococcal toxic shock syndrome
For patients who develop streptococcal toxic shock syndrome, the addition of intravenous immunoglobulin (IVIG) may be considered, but efficacy data are conflicting. Some studies suggest modest benefit; however, one Cochrane review showed no clear benefit on adverse events or mortality.[48]Carapetis JR, Jacoby P, Carville K, et al. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group A streptococcal infections. Clin Infect Dis. 2014 Aug 1;59(3):358-65.
http://www.ncbi.nlm.nih.gov/pubmed/24785239?tool=bestpractice.com
[49]Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome--a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999 Apr;28(4):800-7.
http://www.ncbi.nlm.nih.gov/pubmed/10825042?tool=bestpractice.com
[50]Linnér A, Darenberg J, Sjölin J, et al. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis. 2014 Sep 15;59(6):851-7.
http://www.ncbi.nlm.nih.gov/pubmed/24928291?tool=bestpractice.com
[51]Darenberg J, Ihendyane N, Sjölin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003 Aug 1;37(3):333-40.
http://www.ncbi.nlm.nih.gov/pubmed/12884156?tool=bestpractice.com
[52]Kadri SS, Swihart BJ, Bonne SL, et al. Impact of intravenous immunoglobulin on survival in necrotizing fasciitis with vasopressor-dependent shock: a propensity score-matched analysis from 130 US hospitals. Clin Infect Dis. 2017 Apr 1;64(7):877-85.
http://www.ncbi.nlm.nih.gov/pubmed/28034881?tool=bestpractice.com
[53]Hua C, Bosc R, Sbidian E, et al. Interventions for necrotizing soft tissue infections in adults. Cochrane Database Syst Rev. 2018 May 31;(5):CD011680.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011680.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/29851032?tool=bestpractice.com
Infectious Diseases Society of America (IDSA) guidelines do not include a recommendation regarding the use of IVIG in patients with necrotizing fasciitis with streptococcal toxic shock syndrome, citing the need for additional efficacy studies.[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
World Society of Emergency Surgery consensus recommendations suggest consideration of IVIG in patients with necrotizing fasciitis due to group A streptococcus.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
Choice of antibiotics
Until microbial etiology and antimicrobial susceptibilities are known, empiric broad-spectrum antibiotics should be administered targeting the most common etiologies of type I infection (mixed infections with anaerobes such as Bacteroides or Peptostreptococcus with a facultative anaerobe such as certain Enterobacterales [Escherichia coli, Enterobacter, Klebsiella, Proteus], MRSA, or non-group A streptococcus), and also type II infection due to group A streptococcus. Consider local resistance and epidemiologic patterns (including extended-spectrum beta-lactamase or carbapenemase-producing organisms).
When further information is available and the etiologic agent has been determined, antibiotic therapy should be tailored to target the specific agent. As there are currently no definitive clinical trials, the IDSA recommends continuing antibiotics until no further surgical debridement is needed, the patient has improved clinically, and fever has been absent for 48 to 72 hours.[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
[54]Terzian WTH, Nunn AM, Call EB, et al. Duration of antibiotic therapy in necrotizing soft tissue infections: shorter is safe. Surg Infect (Larchmt). 2022 Jun;23(5):430-5.
https://www.liebertpub.com/doi/10.1089/sur.2022.011
http://www.ncbi.nlm.nih.gov/pubmed/35451883?tool=bestpractice.com
Recommended empiric regimens
Include vancomycin, linezolid, tedizolid, or daptomycin combined with either: piperacillin/tazobactam or a carbapenem (e.g., meropenem, imipenem/cilastatin, ertapenem). Local resistance patterns (including extended-spectrum beta-lactamase or carbapenemase-producing organisms) should be considered. Vancomycin should be used with caution in patients with renal impairment.
Until group A streptococcus involvement is excluded, antimicrobial agents that inhibit toxin production should be included empirically. Clindamycin should be added to empiric treatment until group A streptococcus involvement has been excluded if linezolid is not already being used as part of the empiric regimen.[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
[34]Bonne SL, Kadri SS. Evaluation and management of necrotizing soft tissue infections. Infect Dis Clin North Am. 2017 Sep;31(3):497-511.
http://www.ncbi.nlm.nih.gov/pubmed/28779832?tool=bestpractice.com
Fungal pathogens (especially mucorales) are rare causes of necrotizing fasciitis; empiric inclusion of antifungal agents is not recommended.
Recommendations for type I mixed infections
Include vancomycin, linezolid, tedizolid, or daptomycin combined with either: piperacillin/tazobactam or a carbapenem (e.g., meropenem, imipenem/cilastatin, ertapenem). The IDSA supports some of these regimens.[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
Recommendations for type II infection
Type II infection is most commonly due to group A streptococcus; clindamycin plus penicillin is recommended.[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
For patients with a penicillin allergy, vancomycin monotherapy may be used. When monomicrobial Staphylococcus aureus is the etiologic agent, antibiotics active against MRSA should be used until cultures confirm susceptibilities; options include vancomycin, linezolid, tedizolid, or daptomycin. Ceftaroline, telavancin, or dalbavancin are also reasonable choices, although clinical data are sparse.[2]Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.
https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219-9
http://www.ncbi.nlm.nih.gov/pubmed/30564282?tool=bestpractice.com
[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
Nafcillin, oxacillin, or cefazolin may be used if methicillin susceptibility is confirmed.
Doxycycline is used in the management of type II necrotizing fasciitis attributable to Vibrio vulnificus and Aeromonas hydrophila.
Fungal pathogens are rare causes of necrotizing fasciitis; lipid amphotericin B is the primary treatment option for patients with mucorales infections.
Subsequent management
In treatment-resistant patients, the need for additional debridement or alteration in antibiotic therapy (based on culture results from subcutaneous tissue or blood) should be considered.[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
[5]Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
https://academic.oup.com/cid/article/59/2/e10/2895845
http://www.ncbi.nlm.nih.gov/pubmed/24973422?tool=bestpractice.com
After complete removal of necrotic tissue and final debridement, negative pressure wound therapy may be considered to help with granulation and healing of the wound.[15]Hua C, Urbina T, Bosc R, et al. Necrotising soft-tissue infections. Lancet Infect Dis. 2023 Mar;23(3):e81-94.
http://www.ncbi.nlm.nih.gov/pubmed/36252579?tool=bestpractice.com
Supportive surgical interventions such as fecal diversion for colostomy in cases of Fournier gangrene with fecal contamination, or tracheostomy for patients with cervicofacial necrotizing fasciitis may be warranted.[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
[18]Ord R, Coletti D. Cervico-facial necrotizing fasciitis. Oral Dis. 2009 Mar;15(2):133-41.
http://www.ncbi.nlm.nih.gov/pubmed/19207484?tool=bestpractice.com
Where functional and cosmetic disability results from extensive surgical debridement for necrotizing fasciitis, reconstructive surgery with skin grafting may be required.[3]Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022 Jan 15;17(1):3.
https://wjes.biomedcentral.com/articles/10.1186/s13017-022-00406-2
http://www.ncbi.nlm.nih.gov/pubmed/35033131?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Late signs of necrotizing fasciitis with extensive cellulitis, induration, skin necrosis, and formation of hemorrhagic bullaeFrom: Hasham S, Matteucci P, Stanley PRW, et al. Necrotising fasciitis. BMJ. 2005 Apr 9;330(7495):830-3 [Citation ends].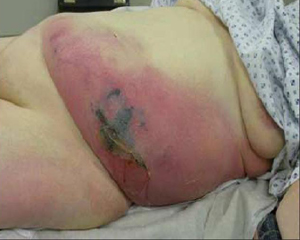
After prolonged hospitalization and recurrent surgical interventions, physical therapy and rehabilitation may be needed for certain patients.
