All patients who are clinically in a state of shock should be managed in a resuscitation, high-dependency, or intensive care setting.
Transfer out of the resuscitation room should only be considered when the patient's vital signs are stabilized. In any evaluation of shock, hemodynamic measurements have to be interpreted in the clinical context of some basic principles. The following questions are useful:
Is the patient in shock? (Is oxygen delivery or cellular metabolic demand not being met?)
Does the patient respond to fluids? (Do they have a decreased preload?)
If the patient is fluid responsive, is the arterial tone (afterload) increased or decreased?
Is pump function (cardiac contractility) increased or decreased?
The answer to these questions will help to determine the type of shock.
[Figure caption and citation for the preceding image starts]: Parameters to differentiate between types of shock and examplesAdapted with permission from "Rady MY. Bench-to-bedside review: Resuscitation in the emergency department. Crit Care. 2005;9:170-176". [Citation ends].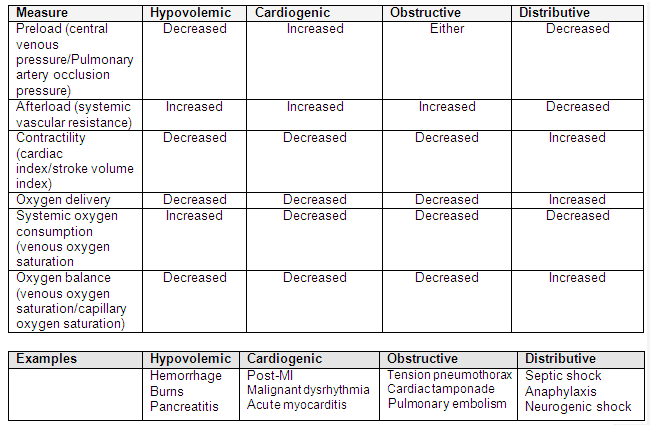
Indicators of regional perfusion, such as arterial or venous serum lactate and base deficit, are important because early hemodynamic assessment based on vital signs and central venous pressure (CVP) does not detect early or persistent global hypoxia.[44]Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996 Mar;14(2):218-25.
http://www.ncbi.nlm.nih.gov/pubmed/8924150?tool=bestpractice.com
Parameters to evaluate shock
Clinical criteria, including vital signs, level of consciousness and assessments of peripheral perfusion (core-periphery temperature gradient, capillary return time) are mandatory.
Hemodynamic monitoring
Ultrasonography[45]Stickles SP, Carpenter CR, Gekle R, et al. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: A systematic review and meta-analysis. CJEM. 2019 May;21(3):406-17.
https://www.cambridge.org/core/journals/canadian-journal-of-emergency-medicine/article/diagnostic-accuracy-of-a-pointofcare-ultrasound-protocol-for-shock-etiology-a-systematic-review-and-metaanalysis/28DF4477E868AC9A1E5A2CD70729FCAF
http://www.ncbi.nlm.nih.gov/pubmed/30696496?tool=bestpractice.com
Decreased inferior vena cava filling and diameter are suggestive of hypovolemic shock.
Point of care abdominal-thoracic ultrasound (e.g, FAST [focused assessment with sonography for trauma]) may reveal pneumothorax (that may be complicated by tension pneumothorax), or free fluid in the abdominal cavity.[46]Stengel D, Leisterer J, Ferrada P, et al. Point-of-care ultrasonography for diagnosing thoracoabdominal injuries in patients with blunt trauma. Cochrane Database Syst Rev. 2018 Dec 12;(12):CD012669.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012669.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/30548249?tool=bestpractice.com
Echocardiography to determine ventricular volumes and cardiac output is helpful in assessing cardiogenic shock.[47]McLean AS. Echocardiography in shock management. Crit Care. 2016 Aug 20;20:275.
https://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1401-7
http://www.ncbi.nlm.nih.gov/pubmed/27543137?tool=bestpractice.com
Cardiac sonography may reveal valvular disorders, or pericardial effusion that may be associated with cardiac tamponade.
Shock Index[48]Rady MY, Nightingale P, Little RA, et al. Shock index: a re-evaluation in acute circulatory failure. Resuscitation. 1992 Jun-Jul;23(3):227-34.
http://www.ncbi.nlm.nih.gov/pubmed/1321482?tool=bestpractice.com
Defined by the ratio of heart rate to systolic BP.
A ratio of <1 is associated with decreased response to volume loading, but if >1, it usually indicates a variable response to fluid administration.[49]Michard F, Ruscio L, Teboul JL. Clinical prediction of fluid responsiveness in acute circulatory failure related to sepsis. Intensive Care Med. 2001 Jul;27(7):1238.
http://www.ncbi.nlm.nih.gov/pubmed/11534577?tool=bestpractice.com
Use of the shock index is mostly confined to hypovolemic shock. It may be unreliable in septic and cardiogenic shock (when heart rate may increase in response to other factors), and in the setting of older age, hypertension, or beta-blocker or calcium-channel blocker therapy.[48]Rady MY, Nightingale P, Little RA, et al. Shock index: a re-evaluation in acute circulatory failure. Resuscitation. 1992 Jun-Jul;23(3):227-34.
http://www.ncbi.nlm.nih.gov/pubmed/1321482?tool=bestpractice.com
[50]Kristensen AK, Holler JG, Hallas J, et al. Is Shock Index a valid predictor of mortality in emergency department patients with hypertension, diabetes, high age, or receipt of β- or calcium channel blockers? Ann Emerg Med. 2016 Jan;67(1):106-13.
http://www.ncbi.nlm.nih.gov/pubmed/26144893?tool=bestpractice.com
Monitoring organ system effects
Urine output of <0.5 mL/kg/hour, and change in mental status and tachypnea, indicate decreased organ perfusion.
Urine output is usually recorded each hour.
Serum markers of tissue metabolism
A normal serum lactate level in a stressed patient is considered to be below 18 mg/dL (2 mmol/L).
Lactate levels >36 mg/dL (>4 mmol/L) have been associated with greater mortality in shock. Early lactate clearance is associated with better prognosis.[51]Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004 Aug;32(8):1637-42.
http://www.ncbi.nlm.nih.gov/pubmed/15286537?tool=bestpractice.com
Lactate can be measured from an arterial or venous gas sample 2 or 3 times a day, or more often if required, to monitor response to treatment.
Base deficit, negative base excess, also correlates with outcome in shock. Initial base deficit does not correlate well with initial blood lactate because there are numerous causes of an elevated lactate (e.g., metformin, beta-2 agonists) other than hypoperfusion. The base excess is defined as the amount of hydrogen ions that would be required to return the pH of the blood to 7.35 if the PaCO₂ levels were adjusted to normal.[52]Jentzer JC, Schrage B, Patel PC, et al. Association between the acidemia, lactic acidosis, and shock severity with outcomes in patients with cardiogenic shock. J Am Heart Assoc. 2022 May 3;11(9):e024932.
https://pmc.ncbi.nlm.nih.gov/articles/PMC9238598
http://www.ncbi.nlm.nih.gov/pubmed/35491996?tool=bestpractice.com
Scoring systems
Several risk stratification approaches have been proposed. All rely on a structured clinical assessment and recording of the patient’s vital signs.[35]National Institute for Health and Care Excellence. Sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
[37]Royal College of Physicians. National Early Warning Score (NEWS) 2. December 2017 [internet publication].
www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2
[38]American College of Emergency Physicians (ACEP) Expert Panel on Sepsis. DART: an evidence-driven tool to guide the early recognition and treatment of sepsis and septic shock [internet publication].
https://poctools.acep.org/POCTool/Sepsis(DART)/276ed0a9-f24d-45f1-8d0c-e908a2758e5a
[40]Surviving Sepsis Campaign. Hour-1 bundle: initial resuscitation for sepsis and septic shock. 2019 [internet publication].
https://sccm.org/getattachment/survivingsepsiscampaign/guidelines/adult-patients/surviving-sepsis-campaign-hour-1-bundle.pdf
It is important to check local guidance for information on which approach your institution recommends. The timeline of ensuing investigations and treatment should be guided by this early assessment.[40]Surviving Sepsis Campaign. Hour-1 bundle: initial resuscitation for sepsis and septic shock. 2019 [internet publication].
https://sccm.org/getattachment/survivingsepsiscampaign/guidelines/adult-patients/surviving-sepsis-campaign-hour-1-bundle.pdf
Sepsis screening tools are designed to promote the early identification of sepsis, and consist of manual methods or the automated use of the electronic health record (EHR). These include the Sequential (or Sepsis-related) Organ Failure Assessment (SOFA) score, the quick SOFA (qSOFA) criteria, National Early Warning Score (NEWS), and Modified Early Warning Score (MEWS). The accuracy of each tool varies, but they are an important component of identifying sepsis early for timely intervention.[36]Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/Fulltext/2021/11000/Surviving_Sepsis_Campaign__International.21.aspx
http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
Of these scoring systems, the SOFA scoring system has been subject to greatest study and has been adopted for use in emergency and critical care management of potential shock victims, including screening for shock and ongoing assessment.[53]Yadav H, Harrison AM, Hanson AC, et al. Improving the accuracy of Cardiovascular Component of the Sequential Organ Failure Assessment Score. Crit Care Med. 2015 Jul;43(7):1449-57.
http://www.ncbi.nlm.nih.gov/pubmed/25785522?tool=bestpractice.com
[54]Cabré L, Mancebo J, Solsona JF, et al; Bioethics Working Group of the SEMICYUC. Multicenter study of the multiple organ dysfunction syndrome in intensive care units: the usefulness of Sequential Organ Failure Assessment scores in decision making. Intensive Care Med. 2005 Jul;31(7):927-33.
http://www.ncbi.nlm.nih.gov/pubmed/15856171?tool=bestpractice.com
[55]Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009 May;37(5):1649-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2703722
http://www.ncbi.nlm.nih.gov/pubmed/19325482?tool=bestpractice.com
A SOFA score of 7 or more on initial evaluation has been associated with significant shock, with a score of 13 or greater associated with significant risk for mortality in the intensive care setting.
[Figure caption and citation for the preceding image starts]: Sequential (or Sepsis-related) Organ Failure Assessment (SOFA) criteriaCreated by BMJ, adapted from Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [Citation ends].
SOFA criteria
The Third International Consensus Group (Sepsis-3) recommends using the SOFA score (primarily validated in patients in intensive care) to assess for sepsis, with a score ≥2 in a patient with a suspected infection being suggestive of sepsis.[34]Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10.
https://jamanetwork.com/journals/jama/fullarticle/2492881
http://www.ncbi.nlm.nih.gov/pubmed/26903338?tool=bestpractice.com
For patients with suspected sepsis outside the intensive care setting, a modified SOFA score, qSOFA, has been found to have better predictability of in-hospital mortality.[56]Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):762-74.
http://www.ncbi.nlm.nih.gov/pubmed/26903335?tool=bestpractice.com
However, there is evidence that qSOFA may have poor sensitivity compared with other bedside early warning scores.[57]Churpek MM, Snyder A, Han X, et al. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017 Apr 1;195(7):906-11.
http://www.ncbi.nlm.nih.gov/pubmed/27649072?tool=bestpractice.com
Therefore, the Surviving Sepsis Campaign advises against using qSOFA, compared with NEWS or MEWS, as a single screening tool for sepsis or septic shock.[36]Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/Fulltext/2021/11000/Surviving_Sepsis_Campaign__International.21.aspx
http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
Early recognition of sepsis is essential because early treatment improves outcomes.[35]National Institute for Health and Care Excellence. Sepsis: recognition, diagnosis and early management. Mar 2024 [internet publication].
https://www.nice.org.uk/guidance/ng51
See urgent considerations section for more details on immediate management.
Measuring the preload
Preload is measured by dynamic response of the CVP to a fluid challenge (e.g., 250 to 500 mL of balanced crystalloid solution). Mini-fluid challenges (e.g., 100 mL) may be used to predict the effects of larger fluid challenges in critically ill patients.[58]Muller L, Toumi M, Bousquet PJ, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology. 2011 Sep;115(3):541-7.
https://pubs.asahq.org/anesthesiology/article/115/3/541/11167/An-Increase-in-Aortic-Blood-Flow-after-an-Infusion
http://www.ncbi.nlm.nih.gov/pubmed/21792056?tool=bestpractice.com
Echocardiography or pulse-induced continuous cardio-output monitoring are also used to determine cardiac output. In selected situations, pulmonary artery catheters may be helpful in initial and ongoing monitoring of preload.
Measuring preload responsiveness
Measures of preload responsiveness can guide fluid administration. Methods include stroke volume variation, systolic pressure variation, pulse pressure variation, plethysmographic variation, and passive leg raising.[59]Hofer CK, Müller SM, Furrer L, et al. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest. 2005;128:848-854.
http://www.ncbi.nlm.nih.gov/pubmed/16100177?tool=bestpractice.com
[60]Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002 Jun;121(6):2000-8.
http://www.ncbi.nlm.nih.gov/pubmed/12065368?tool=bestpractice.com
[61]Cannesson M, Besnard C, Durand PG, et al. Relation between respiratory variations in pulse oximetry plethysmographic waveform amplitude and arterial pulse pressure in ventilated patients. Crit Care. 2005 Oct 5;9(5):R562-8.
https://ccforum.biomedcentral.com/articles/10.1186/cc3799
http://www.ncbi.nlm.nih.gov/pubmed/16277719?tool=bestpractice.com
[62]Natalini G, Rosano A, Taranto M, et al. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: a clinical trial. Anesth Analg. 2006 Dec;103(6):1478-84.
https://journals.lww.com/anesthesia-analgesia/Fulltext/2006/12000/Arterial_Versus_Plethysmographic_Dynamic_Indices.30.aspx
http://www.ncbi.nlm.nih.gov/pubmed/17122227?tool=bestpractice.com
[63]Wyffels PA, Durnez PJ, Helderweirt J, et al. Ventilation-induced plethysmographic variations predict fluid responsiveness in ventilated postoperative cardiac surgery patients. Anesth Analg. 2007 Aug;105(2):448-52.
https://journals.lww.com/anesthesia-analgesia/Fulltext/2007/08000/Ventilation_Induced_Plethysmographic_Variations.28.aspx
http://www.ncbi.nlm.nih.gov/pubmed/17646504?tool=bestpractice.com
[64]Cherpanath TG, Hirsch A, Geerts BF, et al. Predicting fluid responsiveness by passive leg raising: a systematic review and meta-analysis of 23 clinical trials. Crit Care Med. 2016 May;44(5):981-91.
http://www.ncbi.nlm.nih.gov/pubmed/26741579?tool=bestpractice.com
Typically an increase in cardiac output of 10% to 12% after a fluid bolus of 300 to 500 mL of crystalloids is considered to be a positive response.
Evaluation of hemodynamic status is difficult in some situations, including in mechanically ventilated patients. Inappropriate fluid administration can be harmful, and measures of preload (e.g., CVP) are unhelpful because, as end-diastolic pressure exceeds a given value, giving further fluids does not increase stroke volume (Starling principle).[60]Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002 Jun;121(6):2000-8.
http://www.ncbi.nlm.nih.gov/pubmed/12065368?tool=bestpractice.com
Measuring contractility and afterload
Cardiac output is the output of the left ventricle/right ventricle per minute. There are various methods of calculating cardiac output, but an ideal standard has not been established.
The most commonly used method is bedside echocardiography.[47]McLean AS. Echocardiography in shock management. Crit Care. 2016 Aug 20;20:275.
https://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1401-7
http://www.ncbi.nlm.nih.gov/pubmed/27543137?tool=bestpractice.com
Alternative noninvasive methods include Doppler ultrasound and measurements of pulse pressure, although the latter is a function of both cardiac output and arterial function. As cardiac output is affected by the phase of respiration, it needs to be measured at the same point in the respiratory cycle each time to enable comparison.
Other methods include dilution and thermodilution using a pulmonary artery catheter. These use the Fick principle, and the rate at which an indicator substance is diluted or temperature falls is proportional to the cardiac output.
The ratio of the cardiac output (stroke volume x heart rate) to the body surface area in meters squared is the cardiac index. Normal values of the cardiac index range from 2.2 to 2.5 L/minute/m². In cardiogenic shock the cardiac index is typically <1.8 without inotropes and <2.0 to 2.2 with inotropes.[65]Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008 Feb 5;117(5):686-97.
https://circ.ahajournals.org/content/117/5/686.full
http://www.ncbi.nlm.nih.gov/pubmed/18250279?tool=bestpractice.com
Systemic vascular resistance: a measure of afterload derived from the cardiac output, MAP, and CVP.
Measures of tissue perfusion
Oxygen delivery (DO₂): the amount of oxygen delivered to the tissue is calculated as the product of cardiac output, oxygen saturation, and hemoglobin level in the blood. A DO₂ of >600 ml/min/m² has been associated with better outcomes.[66]Pearse R, Dawson D, Fawcett J, et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care. 2005;9(6):R687-93.
http://www.ncbi.nlm.nih.gov/pubmed/16356219?tool=bestpractice.com
Mixed venous oxygen saturations: the saturation of oxygen in the pulmonary artery, and the standard for defining global adequacy of tissue perfusion.[67]Kandel G, Aberman A. Mixed venous oxygen saturation. Its role in the assessment of the critically ill patient. Arch Intern Med. 1983 Jul;143(7):1400-2.
http://www.ncbi.nlm.nih.gov/pubmed/6870412?tool=bestpractice.com
[68]Nelson LD. Continuous venous oximetry in surgical patients. Ann Surg. 1986 Mar;203(3):329-33.
http://www.ncbi.nlm.nih.gov/pubmed/3954486?tool=bestpractice.com
Normal venous saturations are between 65% and 75%. Superior vena cava oxygen saturation is usually higher by about 8% compared with inferior vena cava oxygen saturation, but they parallel each other in shock.[69]Reinhart K, Kuhn HJ, Hartog C, et al. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004 Aug;30(8):1572-8.
http://www.ncbi.nlm.nih.gov/pubmed/15197435?tool=bestpractice.com
[70]Rivers EP, Ander DS, Powell D. Central venous oxygen saturation monitoring in the critically ill patient. Curr Opin Crit Care. 2001 Jun;7(3):204-11.
http://www.ncbi.nlm.nih.gov/pubmed/11436529?tool=bestpractice.com
A sustained elevation of >80% in the presence of low oxygen delivery carries a poor prognosis, as it indicates tissue inability to utilize oxygen, and is usually seen after cardiac arrest resuscitation.[71]Rivers EP, Rady MY, Martin GB, et al. Venous hyperoxia after cardiac arrest: characterization of a defect in systemic oxygen utilization. Chest. 1992 Dec;102(6):1787-93.
http://www.ncbi.nlm.nih.gov/pubmed/1446489?tool=bestpractice.com
During initial stabilization, even after attaining global indices such as BP and urine output, tissue hypoxia can still persist. If uncorrected, it can lead to oxygen debt.[44]Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996 Mar;14(2):218-25.
http://www.ncbi.nlm.nih.gov/pubmed/8924150?tool=bestpractice.com
Oxygen debt is due to an imbalance between supply and demand, which is associated with increased organ dysfunction.[72]Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. JAMA. 1994 Jan 19;271(3):226-33.
http://www.ncbi.nlm.nih.gov/pubmed/8080494?tool=bestpractice.com
Early correction of venous saturations to >70% is associated with better outcomes.[15]Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-77.
https://www.nejm.org/doi/full/10.1056/NEJMoa010307#t=article
http://www.ncbi.nlm.nih.gov/pubmed/11794169?tool=bestpractice.com
Venous saturations of <70% are an independent predictor of mortality.[73]Varpula M, Tallgren M, Saukkonen K, et al. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005 Aug;31(8):1066-71.
http://www.ncbi.nlm.nih.gov/pubmed/15973520?tool=bestpractice.com
Lactate: levels >36 mg/dL (>4 mmol/L) are associated with greater mortality in shock. Base excess levels are used during bedside resuscitation in determining fluid volume replacement for acute trauma victims.

