Tests
1st tests to order
mammogram
Test
Bilateral diagnostic mammography should be used as the initial imaging test to evaluate symptomatic adult patients ages ≥30 years or as follow-up to evaluate abnormal findings on screening mammography or other imaging tests.[101][129][130]
The sensitivity and specificity of conventional mammography for diagnosing breast lesions have been reported as 78.9% and 82.7%, respectively.[131]
Digital breast tomosynthesis (DBT) is a three-dimensional mammographic technique that can be used to create thin-section reconstructed images of breast tissue. Diagnostic DBT may offer improved detection and lesion characterization compared with conventional two-dimensional mammography, especially in patients with dense breast tissue.[129] In one study, DBT showed a higher overall sensitivity, compared with conventional mammography (88.2% vs. 78.3%, respectively), with a similar specificity.[132]
Diagnostic conventional mammography or diagnostic DBT may be used as alternative options or in combination.[130][133] National Comprehensive Cancer Network (NCCN) guidelines recommend DBT alongside conventional diagnostic mammography.[101] Contrast-enhanced mammography may be a further option for initial diagnostic imaging.[101][133]
If a mammogram does not discover an abnormality in patients who have a clinically detected breast mass, additional imaging (e.g., ultrasound or MRI) should be performed for further evaluation.
The sensitivity of conventional mammography is lower in women with dense breasts; therefore, DBT, or supplemental ultrasound or MRI may be warranted in these women.[101][134][135]
Result
findings suggestive of malignancy include: an irregular spiculated mass, clustered microcalcifications, and linear branching calcifications
Tests to consider
breast ultrasound
Test
Evaluation of a new mass in a woman aged <30 years should usually begin with ultrasound. However, if there is a high suspicion of malignancy (e.g., based on personal or family history, clinical breast exam, or ultrasound results), a mammogram should be performed first. If there is a low clinical suspicion, observation of the mass for 1-2 menstrual cycles may be considered, followed by ultrasound or mammography if symptoms persist.[101]
For women ages ≥30 years with palpable symptoms, and patients with other suspicious symptoms at any age, ultrasound may be performed in addition to diagnostic mammography and/or DBT.[101][130][133][143]
Breast ultrasound has demonstrated utility as an adjunct to mammography through specificity (by differentiating cysts from solid masses), evaluating breast or axillary masses that are not sufficiently assessed by mammogram, evaluating axillary lymph nodal involvement, and monitoring for tumor response during neoadjuvant chemotherapy.[136][137][138][139]
The sensitivity and specificity of ultrasound for diagnosing breast lesions has been reported to be 88.9% and 77.9%, respectively.[131] The sensitivity and specificity of the combination of ultrasound and mammography for diagnosing breast lesions has been reported as 94.2% and 67.9%, respectively.[131]
Three-dimensional breast ultrasound has a sensitivity of 92.3% and a specificity of 87.2% for diagnosing breast cancer in women with breast nodules or mass lesion.[140]
Result
findings suggestive of malignancy include: a hypoechoic mass, an irregular mass with internal calcifications, and enlarged axillary lymph nodes
breast MRI
Test
Breast MRI (without and with contrast) may be useful for evaluation and decision-making in certain circumstances:
Evaluation of suspicious nipple discharge, inversion, or retraction, or suspicious breast skin changes when ultrasound or mammography are not diagnostic. MRI may also facilitate a diagnosis of inflammatory breast cancer;[101]
staging evaluation (to define extent of cancer or presence of multifocal or multicentric cancer), if indicated, or to screen for cancer in the contralateral breast at diagnosis;[116][143]
evaluation before and after preoperative systemic therapy (to define the extent of disease, response to treatment, and potential for breast-conserving surgery);[116][143]
identifying occult primary tumors in patients with clinically positive axillary nodes; or with Paget disease (to define the extent of disease); or with invasive lobular carcinoma (not adequately identified on mammography, ultrasound, or physical exam).[101][116]
Sensitivity of breast MRI is higher than for mammogram, but specificity is limited (sensitivity for breast cancer is approximately 88% to 91%; specificity is approximately 68%.[141] Despite increased sensitivity, MRI has not been shown to decrease rates of reoperation when added to the workup of women with primary breast cancer undergoing wide local excision.[142] Breast MRI is not recommended routinely for diagnostic evaluation after a diagnosis of breast cancer, because of the risk of false positives and potential for overtreatment.[143][144]
Result
findings suggestive of malignancy include: a heterogeneously enhancing area and significant architectural distortion
biopsy
Test
Biopsy is required for definitive diagnosis.
An image-guided core biopsy is usually the preferred method of diagnosis because it enables differentiation between preinvasive and invasive disease, is less likely to be associated with inadequate sampling, and enables assessment of receptor status. [Figure caption and citation for the preceding image starts]: Inflammatory breast carcinoma showing dermal lymphatic invasion by tumor cellsFrom the collection of Dr Massimo Cristofanilli; used with permission [Citation ends].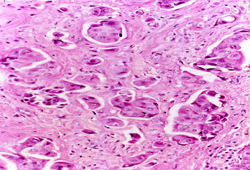
Excisional biopsy may be indicated if core needle biopsy cannot be performed; or results are indeterminate, or are benign and discordant with imaging; or where larger tissue samples are needed. However, it is associated with poorer cosmesis than needle biopsy, is more costly, and requires surgery.[116]
Fine needle aspiration (FNA) is useful in obtaining a rapid diagnosis of breast malignancy, and it may be the only test required for diagnosis when plans for immediate surgery are already in place. Sensitivity and specificity of FNA are reported to be 98% and 97%, respectively, when performed by experienced clinicians.[146] However, diagnostic accuracy with FNA is likely to decline if performed by less experienced clinicians.
Result
histologic findings confirming an invasive ductal carcinoma, invasive lobular carcinoma, medullary carcinoma, mucinous carcinoma, or metaplastic carcinoma
hormone receptor testing
Test
Determination of the estrogen receptor (ER) and progesterone receptor (PR) status should be performed once a diagnosis of invasive breast cancer has been made.[147][148]
ER and PR status is assayed using immunohistochemistry.
The American Society of Clinical Oncology and the College of American Pathologists recommend that ER and PR assays should be considered positive if there are at least 1% positive tumor nuclei in the initial biopsy sample.[147]
Women who are ER-borderline are managed in the same way as women who are ER-positive.
Result
positive or negative
HER2 testing
Test
Patients diagnosed with breast cancer (early-stage or metastatic disease) should have at least one tumor sample tested for HER2 expression.[148]
A HER2 test includes testing for HER2 protein expression (immunohistochemistry [IHC] assay) or HER2 gene amplification by in situ hybridization (ISH).[148]
IHC scoring ranges from 0 to + 3+ as determined by intensity of staining, and percentage (>10%) of contiguous and homogeneous positive tumor cells. HER2 status can be classified as follows, based on the IHC score: HER2 negative (IHC score 0 or 1+); equivocal (IHC score 2+ [requires reflex testing with ISH assay]); or HER2 positive (IHC score 3+).[148]
Single-probe ISH assays measure the average HER2 copy number (signals/cell); dual-probe ISH assay measures the HER2/CEP17 ratio.[148] The single-probe approach is not preferentially recommended; if used, cases with average HER2 copy number ≥4.0 and <6.0 signals/cell should base final results on concurrent IHC and if 2+ reflexed to dual-probe ISH testing.[116]
Assuming no apparent histopathologic discordance observed by the pathologist, HER2 status can be classified as negative or positive, based on concurrent IHC and ISH results.[148]
Patients with the following IHC and ISH (dual probe) results are HER2 negative: HER2/CEP17 ratio <2.0 AND average HER2 copy number <4.0 signals/cell (no concurrent IHC result required); or HER2/CEP17 ratio ≥2.0 AND average HER2 copy number <4.0 signals/cell and concurrent IHC score 0, 1+, or 2+; or HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥6.0 signals/cell and concurrent IHC score 0 or 1+; or HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥4.0 and <6.0 signals/cell and concurrent IHC score 0, 1+, or 2+.[148]
Patients with the following IHC and ISH (dual probe) results are HER2 positive: HER2/CEP17 ratio ≥2.0 AND average HER2 copy number <4.0 signals/cell and concurrent IHC score 3+; or HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥6.0 signals/cell and concurrent IHC score 2+ or 3+; or HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥4.0 and <6.0 signals/cell and concurrent IHC score 3+; or HER2/CEP17 ratio ≥2.0 AND average HER2 copy number ≥4.0 signals/cell (no concurrent IHC result required).[148]
Result
positive or negative
gene expression assays
Test
Gene expression assays may be used for prognostication and to guide decisions on adjuvant chemotherapy.[151][152][153][154][155]
Oncotype DX® is the preferred assay to determine whether the addition of chemotherapy to endocrine therapy would be of benefit in patients with HR-positive, HER2-negative disease who are node-negative or postmenopausal with node-positive disease (1-3 positive nodes).[116][155][156]
Oncotype DX® is a reverse transcription polymerase chain reaction-based multigene assay that evaluates the expression of 21 genes within the patient's paraffin-embedded tumor slides.[157] Based on this expression, a low (≤10), intermediate (11-25), or high (26-100) recurrence score can be calculated. The recurrence score can aid decision-making on whether a patient with hormone receptor-positive breast cancer who is node-negative or positive for 1-3 nodes would benefit from adjuvant chemotherapy, or if adjuvant endocrine therapy alone would be sufficient.[151][158][159][160]
Premenopausal patients with 1-3 positive nodes benefit from chemotherapy regardless of genomic assay result. The clinical utility of assays in node-positive disease with ≥4 nodes is unknown.[155]
Other assays, such as Mammaprint®, Breast Cancer Index (BCI), Prosigna®, and EndoPredict®, may be used to provide prognostic information in postmenopausal women or women ages >50 years who are node negative. Mammaprint® and EndoPredict® may also be used for postmenopausal women or women ages >50 years with 1-3 positive nodes. However, the ability of these assays to predict therapeutic benefit is less certain.[116][155]
The TAILORx study assessed the efficacy of Oncotype DX® in women with hormone receptor-positive, HER2-negative, node-negative breast cancer. The study reported a low rate of distant recurrence (3%) at 9 years of follow-up in those with an Oncotype DX® recurrence score ≤10 (low risk) who received adjuvant endocrine therapy alone.[151][158] In those with an Oncotype DX® recurrence score of 11-25 (intermediate risk), adjuvant endocrine therapy alone was found to be noninferior to adjuvant chemotherapy plus endocrine therapy at 9 years of follow-up.[158] However, in a subanalysis, use of adjuvant chemotherapy was found to be beneficial in women ages ≤50 years with a recurrence score of 16-25.[158] In those with an Oncotype DX® recurrence score of 26-100 (high risk) who received adjuvant chemotherapy plus endocrine therapy, the estimated rate of freedom of distant recurrence at 5 years of follow-up was greater than would be expected if endocrine therapy alone was used in this high-risk group.[159]
Result
variable genomic profile and recurrence risk score
computed tomography
Test
Routine systemic staging (CT, PET/CT, and bone scan) is not indicated for early-stage breast cancer in the absence of signs or symptoms of metastasis.[116][145] In women with symptoms or signs suggestive of metastatic disease or those with locally advanced disease (T3, N1-2, M0), additional imaging should be considered.[143]
Pulmonary symptoms should be investigated with a chest CT (without or with contrast). Abdominal and pelvic imaging using CT with contrast or MRI with contrast is indicated for abdominal or pelvic symptoms or signs, elevated alkaline phosphatase, or abnormal liver function tests.[116]
Positron emission tomography (PET)/CT scan is not indicated in stage I, stage II, or operable stage III breast cancer because of its high false-negative rate for small lesions, low sensitivity for detection of axillary lymph node metastases, and high rate of false-positive scans. PET/CT is most helpful in advanced disease and invasive ductal histology when standard staging investigations are equivocal.[116][133][143]
Result
may show pulmonary or abdominal metastases
bone scan
Test
Routine systemic staging (CT, PET/CT, and bone scan) is not indicated for early-stage breast cancer in the absence of signs or symptoms of metastasis.[116][145] In women with symptoms or signs suggestive of metastatic disease or those with locally advanced disease (T3, N1-2, M0), additional imaging should be considered.[143] Radionuclide bone scan should be considered in patients with localized bone pain or elevated alkaline phosphatase.[116]
Result
may show abnormal bone lesions indicating metastases
genetic testing
Test
Performed to detect germline mutations associated with increased cancer risk. It is estimated that 5% to 10% of breast cancers are linked to inherited genetic mutations.[11][12][13] BRCA1 and BRCA2 mutations are the most common inherited genetic mutations found in breast cancer.[13][14][15][40]
Genetic testing can inform prognosis, and aid in systemic therapy and surgical decision-making, (e.g., adjuvant olaparib treatment for high-risk, HER2-negative breast cancer; risk-reducing surgery).[17]
Genetic counseling and testing for high-penetrance breast cancer susceptibility genes is recommended for certain patients at diagnosis based on personal or family history, ancestry, diagnosis at an early age, or eligibility for olaparib therapy.[17][116][149]
All male patients with breast cancer at any age should have genetic testing.[17][116][149]
National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing for high-penetrance breast cancer susceptibility genes (e.g., BRCA1, BRCA2, CDH1, PALB2, PTEN, STK11, and TP53) at diagnosis for the following patients: diagnosed at ages ≤50 years; with Ashkenazi Jewish ancestry and diagnosed at any age; males diagnosed at any age; with triple-negative breast cancer, or multiple primary (synchronous or metachronous) breast cancers, or lobular breast cancer (with a personal or family history of diffuse gastric cancer) diagnosed at any age; candidate for adjuvant olaparib therapy; with any blood relative with a known pathogenic/likely pathogenic variant in a cancer susceptibility gene; with a strong family history, including: ≥1 close blood relative diagnosed with breast cancer at ages ≤50 years, or with male breast cancer, ovarian or pancreatic cancer, or prostate cancer (with metastatic, or high- or very high-risk group) at any age; or ≥3 diagnoses of breast and/or prostate cancer on the same side of the family (including the patient being assessed).[17][116]
The American Society of Clinical Oncology (ASCO) found that expanding the NCCN age criteria to include all women ≤65 years improved the sensitivity of the criteria (to 98% for BRCA1 or BRCA2). ASCO recommends germline testing for BRCA1 and BRCA2 mutations at diagnosis in the following patients: diagnosed with breast cancer at ages ≤65 years; select patients >65 years diagnosed with breast cancer, based on personal or family history, ancestry, or eligibility for olaparib therapy.[149]
ASCO guidelines recommend individualized testing for additional high-penetrance genes (e.g., CDH1, PALB2, PTEN, STK11, and TP53) based on personal or family history.[149]
Genetic testing for a specific pathogenic variant can be carried out, if known; germline multigene panel testing is recommended if the variant is unknown.[17] Selection of the specific multigene panel should take into account the patient's personal and family history.[149]
If there are existing genetic test results, do not order a duplicate test unless there is uncertainty about the existing result, for example the result is inconsistent with the patient’s clinical presentation, the test methodology has changed, or new mutations have been identified since last testing.[150]
Result
may show gene mutation associated with increased cancer risk, for example, BRCA1, BRCA2, CDH1, PALB2, PTEN, STK11, and TP53)
CBC
Test
Blood tests are not generally recommended as part of staging and preoperative workup.
A CBC, a comprehensive metabolic panel, liver function tests, and an alkaline phosphatase test should be considered only if the patient is a candidate for preoperative or adjuvant systemic therapy.[116]
Patients with a clinical/pathologic diagnosis of inflammatory breast cancer without distant metastasis should have a CBC and platelet count.[116]
Result
may show anemia, thrombocytopenia, leukopenia/neutropenia
LFTs
Test
Blood tests are not generally recommended as part of staging and preoperative workup.
A CBC, a comprehensive metabolic panel, liver function tests, and an alkaline phosphatase test should be considered only if the patient is a candidate for preoperative or adjuvant systemic therapy.[116]
Patients with a clinical/pathologic diagnosis of inflammatory breast cancer without distant metastasis should have a CBC and platelet count.[116]
Result
may be elevated
alkaline phosphatase
Test
Blood tests are not generally recommended as part of staging and preoperative workup.
A CBC, a comprehensive metabolic panel, liver function tests, and an alkaline phosphatase test should be considered only if the patient is a candidate for preoperative or adjuvant systemic therapy.[116]
Patients with a clinical/pathologic diagnosis of inflammatory breast cancer without distant metastasis should have a CBC and platelet count.[116]
Result
may be elevated
Use of this content is subject to our disclaimer