Approach
When evaluating a patient with a concern of memory loss, the following questions should be considered:
Does the patient truly have memory loss or is there another cognitive problem causing the memory disorder?
What is the localization within the brain of the memory problem?
What is the temporal profile for the memory loss: acute (seconds/minutes/hours), subacute (days/weeks), or chronic (many months to years)?
What etiologies could be responsible for the memory disorder?
Memory loss versus another cognitive problem
The first consideration is to determine whether the patient truly has memory loss or another cognitive problem.
Some degree of memory loss occurs normally with aging. Normal aging leads to decreased ability for retrieval from a decline in frontal lobe function, but does not impact the activities of daily living.[39] Memory loss becomes particularly concerning when it affects function or activities of daily living.
Memory dysfunction can result from hippocampal lesions (short-term memory loss, e.g., Alzheimer dementia) as well as from lesions of brain structures involved in long-term storage (e.g., semantic dementia). In many cases, memory is encoded properly in the hippocampus, but patients have trouble retrieving the stored memory. This retrieval deficit is typically due to problems with frontal lobe function, often caused by white matter disease.
It is important to differentiate transient, fluctuating disturbances in consciousness due to a delirium from an underlying memory disorder. Many medications can induce confusion or even delirium in the elderly; for example, the greater the number of anticholinergic medications a patient is taking, the greater the risk of hospitalization for confusion or dementia.[40]
The history, exam, and neuropsychological testing can all be helpful in distinguishing a primary memory disorder from a delirium or impairment in retrieval.
Pseudodementia is a descriptive term for a cognitive deficit secondary to a primary psychiatric disturbance, which mimics dementia and is reversible once the primary diagnosis has been treated.[41] Such primary conditions include depressive disorder, bipolar disorder, and schizophrenia. Pseudodementia may result in a clinical presentation suggestive of a memory disorder with diminished concentration, sleep disruption, and mild impairments on delayed recall.[39] A geriatric depression scale is often used to help differentiate between depressive pseudodementia and other forms of dementia.[42] [ Geriatric Depression Scale Opens in new window ] Results from the scale are combined with other information about a person's history and current functioning to help with diagnosis.
Patients with memory problems or complaints who do not fit a diagnosis of dementia because they are not functionally impaired are often referred to as having mild cognitive impairment (MCI). The deficits in patients with MCI, by definition, are not severe enough to interfere with their normal activities of daily living.
Localization of memory problem
The next step in evaluating a patient with memory loss is determining the localization of the memory loss. Memory has traditionally been divided into the following memory systems: episodic, working, semantic, and procedural memory.[43] Separate brain structures are responsible for each of these memory types.
Episodic memory: lasts minutes to years, and localizes to the hippocampus and limbic circuits.[43]
Working memory: a type of memory lasting seconds and involving active rehearsal of information; relies on the integrity of the prefrontal cortex, and the echoic memory in the angular gyrus.[43]
Semantic memory: typically consists of factual information (e.g., knowing what a certain object is and what it is used for, knowing who was the first President of the US), and localizes to the inferolateral temporal lobes.[43]
Procedural memory: pertains to driving a car or riding a bike, and involves the basal ganglia, cerebellum, and supplementary motor area.[43]
The type of memory impairment manifested through the history, physical exam, and neuropsychological testing can give an indication of the localization of the disease process.
Temporal profile
The rate of clinical progression of memory loss is a helpful clue to understanding the underlying etiology:
Acute symptoms (i.e., within minutes to hours) are suggestive of ischemic stroke, hemorrhagic stroke, seizures, migraine, or transient global amnesia.[Figure caption and citation for the preceding image starts]: Diagnostic algorithm for acute onset memory deficit. 1: changes in signal intensities, structural lesions on brain MRI; 2: sometimes increased signal intensities on diffusion-weighted imaging; EEG, electroencephalogram; MRI, magnetic resonance imagingCreated by Mee-Ohk Kim, MD, PhD and Michael D. Geschwind, MD, PhD; used with permission [Citation ends].

Subacute symptoms (i.e., within days to months) typically occur in infectious and inflammatory conditions, such as limbic encephalopathy and Wernicke-Korsakoff syndrome. Dementia that progresses over weeks to months occurs in Creutzfeldt-Jakob disease (CJD) and Hashimoto encephalopathy.[Figure caption and citation for the preceding image starts]: Diagnostic algorithm for subacute onset memory deficit. 1: changes in signal intensities, structural lesions on brain MRI; 3: MRI in CJD shows cortical ribboning and/or deep nuclei restricted diffusion on diffusion-weighted imaging/apparent diffusion coefficient map; CSF biomarkers might be elevated; ADC, apparent diffusion coefficient; CJD, Creutzfeldt-Jakob disease; CSF, cerebrospinal fluid; DWI, diffusion-weighted imaging; HSV, herpes simplex virus; MRI, magnetic resonance imaging; NSE, neuron-specific enolaseCreated by Mee-Ohk Kim, MD, PhD and Michael D. Geschwind, MD, PhD; used with permission [Citation ends].

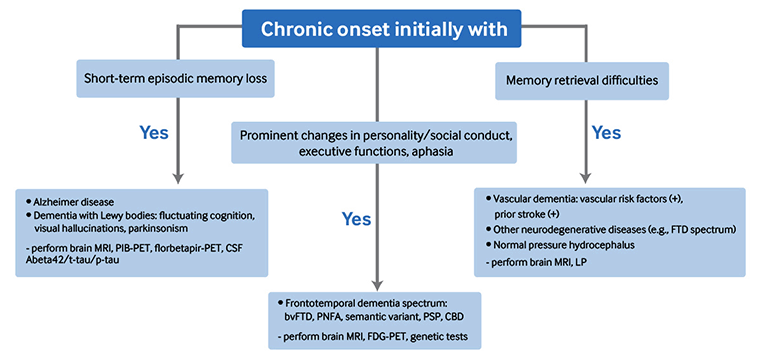
Chronic symptoms (>2 years) are suggestive of a neurodegenerative process or vascular dementia. Memory loss from small vessel ischemic vascular disease is often gradual and not stepwise. [Figure caption and citation for the preceding image starts]: Diagnostic algorithm for chronic onset memory deficit; bvFTD, behavioral variant frontotemporal dementia; CBD, corticobasal degeneration; FDG-PET, fluorodeoxyglucose - positron emission tomography; FTD, frontotemporal degeneration; LP, lumbar puncture; PIB-PET, Pittsburgh compound B - positron emission tomography; PNFA, progressive nonfluent aphasia; PSP, progressive supranuclear palsyCreated by Mee-Ohk Kim, MD, PhD and Michael D. Geschwind, MD, PhD; used with permission [Citation ends].
History
A comprehensive history is the first and most essential step of the diagnostic workup of memory loss. As many patients may be unaware of their deficits, reliable informants who interact with the patient on a regular (ideally daily) basis are the key to a complete history. The history will help delineate the time course, initial symptoms, and any pathognomonic symptoms accompanying the memory loss.
Basic screening questions for other cognitive domains in addition to memory (e.g., executive, language, visuospatial, behavior, mood, motor, and autonomic system) will further differentiate the various possible causes of neurodegenerative disease. Finally, accompanying symptoms that occur in addition to the memory loss should be screened for. These features may help to distinguish the various causes of memory impairment.
Presenting symptoms
Short-term episodic memory loss (e.g., repetitive questioning, misplaced belongings) is a common early symptom in Alzheimer dementia (AD).
Visuospatial dysfunction (e.g., becoming lost in unfamiliar places) occurs in AD. Visuospatial dysfunction is a prominent symptom of dementia with Lewy bodies.
Language problems: patients with early AD may have difficulty speaking and difficulty naming things. Later in the disease, patients can develop nonfluent speech, paraphrasing, and conveying information inappropriately.
Significant personality and social behavior changes occur early in the course of frontotemporal dementia and CJD, but much later in the course of AD. Personality changes also occur in progressive supranuclear palsy (PSP) and behavioral changes occur in limbic encephalopathy.
Cognitive changes in vascular dementia are variable and highly dependent on the particular area of the brain affected by the vascular pathology. Attention, information processing, and executive function deficits are common.[44] Difficulty initiating activity, slowed cognitive processing, difficulty problem solving, perseveration, and disinhibition may all occur in vascular dementia.
Long-term memory loss is a late feature of AD.
Prosopagnosia and autoprosopagnosia can occur late in AD.
Awareness: fluctuating awareness occurs in dementia with Lewy bodies. Altered awareness can be a feature of limbic encephalopathy. Rapid-onset altered consciousness is a feature of Hashimoto encephalopathy. Repeated, stereotyped episodes of altered consciousness characterize complex seizures.
Hallucinations: complex visual hallucinations point to dementia with Lewy bodies. Hallucinations and delusions may be present in vascular dementia. Hallucinations also occur in Hashimoto encephalopathy.
Depression, apathy, and anxiety are common early symptoms of corticobasal degeneration. Patients with AD may have symptoms of apathy and depression earlier in the disease course, before there are more significant personality changes. Depression may occur in patients with vascular dementia.
Corticobasal degeneration can begin with cognitive symptoms, including aphasia.[7]
Anterior thalamic infarcts might result in chronic symptoms of apathy or perseveration and memory loss.
Irritability, apathy, somnolence, suspiciousness, emotional instability, and confusion may occur in vitamin B12 deficiency.
Patients with semantic dementia lose knowledge of what things are (semantics). They may also experience an inability to recognize emotions on the faces of others and to empathize.
Associated symptoms
Motor symptoms: generalized tonic-clonic motor activity occurs in generalized tonic-clonic seizures. Impaired downward gaze and neck and trunk stiffness occur in PSP. Parkinsonism (e.g., bradykinesia, rigidity, masked facies, tremor, stooped posture, gait disturbances) is found in a variety of neurodegenerative diseases, including dementia with Lewy bodies, CJD, PSP, and corticobasal degeneration.
Gait disorders occur in normal pressure hydrocephalus. Patients with vascular dementia might have slowing of gait, shuffling, and feet stuck to the floor when commencing walking. Ataxia might be seen in many dementias, including CJD, HIV dementia, limbic encephalopathy (and various autoimmune paraneoplastic disorders), Hashimoto encephalopathy, ischemic stroke/vascular dementia, vitamin B12 deficiency, and Wernicke-Korsakoff syndrome.
Falls: unexplained falls occur early in the disease course of PSP.
Limb and/or facial weakness, paresthesia, numbness, and speech difficulty may occur in anterior circulation stroke. Hashimoto encephalopathy can cause stroke-like symptoms.
Dizziness can be a feature of posterior circulation stroke or can occur after traumatic brain injury.
Visual loss or double vision can occur in a posterior circulation stroke.
Headache: sudden onset of severe headache can occur in hemorrhagic stroke. In migraine, episodes of repeated unilateral, throbbing headache, which may or may not be accompanied by a visual aura, occur. Headache may be a sign of raised intracranial pressure in patients with a mass lesion. Headache may also occur following traumatic brain injury.
Nausea and vomiting can occur in migraine and, rarely, in transient global amnesia. Patients with raised intracranial pressure caused by a mass lesion may report nausea and vomiting.
Phonophobia may occur in migraine and following traumatic brain injury.
Photophobia may occur in migraine.
Muscle wasting and weakness are suggestive of amyotrophic lateral sclerosis, which can occur with frontotemporal dementia.
REM sleep disturbances (e.g., jerking or complex movements during sleep) occur in dementia with Lewy bodies.
Seizures may occur in limbic encephalopathy and Hashimoto encephalopathy.
Dysphagia occurs in PSP.
Dysarthria is seen in PSP, corticobasal degeneration, disorders affecting the cerebellum, and stroke.
Hypophonia (soft voice) occurs in dementia with Lewy bodies and PSP.
Urinary incontinence occurs in normal pressure hydrocephalus and may occur during seizures.
Fatigue may occur in hypothyroidism and following traumatic brain injury.
Cold intolerance, hair loss, weight gain, and neuropsychiatric symptoms sometimes occur in hypothyroidism.
Past medical history
Hypertension, diabetes, hypercholesterolemia, ischemic heart disease, and/or history of stroke are often present in patients with vascular dementia or acute ischemic stroke. One large cohort study found that systolic blood pressure ≥130 mmHg at age 50 is associated with dementia, and this excess risk is independent of cardiovascular disease.[45]
Risk factors for hemorrhagic stroke include hypertension, amyloid angiopathy, venous sinus thrombosis, hemorrhagic tumor, and arteriovenous malformation.
Frontotemporal dementia is often associated with amyotrophic lateral sclerosis.
Patients with autoimmune thyroiditis may develop Hashimoto encephalopathy, which is associated with markedly elevated anti-thyroperoxidase or anti-thyroglobulin antibodies.
Patients with neurosyphilis might have a distant history of genital lesion or palmar rash.
Risk factors for seizures may be present. These include: prior history of head injury, developmental delay, infantile febrile seizures, prior meningoencephalitis, and intraparenchymal mass lesions.
Wernicke-Korsakoff syndrome results from thiamine deficiency, which can be caused by the following: alcoholism, bariatric surgery, or malnutrition.
Patients with vitamin B12 deficiency often have risk factors for deficiency (e.g., strict vegan diet without vitamin supplements, malnutrition, pernicious anemia, extensive bowel surgery, institutionalization, anemia, gastric atrophy).
Lung, breast, colorectal and kidney cancer, and melanoma may metastasize to the brain.
Family history
A family history of at least three generations is helpful to identify patients who might have an increased risk of AD. Specifically enquire about age of onset of any neurologic or psychiatric symptoms, type of dementia and method of diagnosis, current ages of family members, age at death for deceased relatives (especially unaffected relatives), and causes of death.[46] Around 10% to 15% of cases of CJD are familial.[47]
Drug history
Medications with anticholinergic properties can exacerbate symptoms of memory deficit.[48] Many other drugs can affect cognition, including benzodiazepines, opioids, antipsychotics, corticosteroids, some anticonvulsants, and some antidepressants.[49]
Patients may be concerned about a link between taking statins and cognitive decline. However, one large Australian prospective cohort study found no difference in the rate of decline in memory and global cognition over 6 years between statin users and never users.[50]
Social history
Establishing the impact that the symptoms are having on the patient’s daily life is essential. The patient’s symptoms may jeopardize their safety, for example if they forget to turn off cooking appliances, or wander off and are then unable to find the way home. Enquire about washing, feeding, toileting, shopping, cooking, managing finances, remembering appointments and traveling.[51]
Ask whether the patient drives a vehicle. In some countries, such as the UK, patients must inform regulatory bodies of severe memory problems or a diagnosis of dementia.[52]
Positive HIV risk factors (intravenous drug use, unprotected sex, needlestick injury) may be present in HIV dementia. Symptoms usually develop over months, although they may have a rapid onset during seroconversion. Patients may experience fluctuations in cognition or apathy.
Transient global amnesia is frequently preceded by stress, excessive physical activity, or an emotional event, and resolves spontaneously over hours.
Stress, menstruation, sleep deprivation, and certain foods may trigger migraines.
Collateral history
History from a reliable informant who is familiar with the patient is an invaluable part of the assessment. One possible tool is the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), which is an informant-based (e.g., family, friends, etc.) questionnaire about changes in the cognitive function of their elderly loved ones. The sensitivity and specificity of IQCODE to diagnose dementia in community settings is approximately 80%.[53] When the IQCODE was used in the general hospital setting or in memory clinics, the combined sensitivity was 0.91 (95% CI 0.86 to 0.94) and specificity was 0.66 (95% CI 0.56 to 0.75). Use of the test in the general hospital setting, however, provided much greater accuracy (sensitivity 0.95 [95% CI 0.88 to 0.98] vs. 0.90 [95% CI 0.83 to 0.94]; specificity 0.81 [95% CI 0.71 to 0.88] vs. 0.54 [95% CI 0.44 to 0.64]).[54]
Other informant-based tools for detecting dementia and/or delirium might include the AD8 test and the Dementia=(MC)² test.[55]
Neurologic exam
The second part of the workup is the neurologic exam. Different findings on neurologic examination can be helpful in the differential diagnosis of memory deficit.
Mental status
Impaired arousability is frequently seen in dementia with Lewy bodies, but may also be noted in other disorders, such as HIV dementia, limbic encephalopathy, Hashimoto encephalopathy, traumatic brain injury, hypothyroidism, normal pressure hydrocephalus, and hypoxic brain injury secondary to cardiac arrest.
Aphasia (language dysfunction) is seen in vascular dementia, semantic dementia, CJD, PSP, corticobasal degeneration, HIV dementia, ischemic/hemorrhagic stroke, and traumatic brain injury.
Apraxia (loss of ability to carry out learned movements) is seen in CJD, vascular dementia, corticobasal degeneration, and Alzheimer disease.
Patients with frontotemporal dementia often have poor comportment.
Cranial nerve exam
Papilledema might be noted in traumatic brain injury or any process causing brain edema, such as a tumor.
Sixth cranial nerve palsy may occur in raised intracranial pressure. The nerve is stretched during its long intracranial course.
Ophthalmoplegia may be seen in PSP, corticobasal degeneration, mitochondrial disorders, or Wernicke-Korsakoff syndrome.
Predominant wide-eyed stare and furrowed brow is characteristic of PSP.
Nystagmus is seen in Wernicke-Korsakoff syndrome.
Impairment of visual smooth pursuit is seen in HIV dementia and other disorders.
Pupils constrict to accommodation but not light. Argyll Robertson pupils might be seen in patients with neurosyphilis. Homonymous hemianopsia is seen in vascular dementia, stroke, CJD, or posterior cortical atrophy syndromes (usually due to Alzheimer or dementia with Lewy bodies).
Dysarthria is seen in PSP, corticobasal degeneration, disorders affecting the cerebellum, and stroke.
Hypophonia (soft voice) occurs in dementia with Lewy bodies and PSP.
Motor exam
Myoclonus is seen in dementia with Lewy bodies, CJD, corticobasal degeneration, limbic encephalopathy, Hashimoto encephalopathy, and hypoxic injury.
Hemiparesis or hemiplegia and hemisensory loss are most often seen in vascular dementia (stroke patients). Focal weakness occurs in traumatic brain injury if the resulting lesion (hematoma) impinges upon the corticospinal tracts. Signs of stroke might also be seen in neurosyphilis.
Parkinsonism (e.g., bradykinesia, rigidity, masked facies, tremor, stooped posture, gait disturbances) is found in a variety of neurodegenerative diseases, including dementia with Lewy bodies, CJD, PSP, and corticobasal degeneration.
Limb dystonia, mirror movements, and alien limb phenomenon are seen in corticobasal syndrome, which is often due to corticobasal degeneration or Alzheimer disease, and CJD.[7]
Sensory exam
Peripheral neuropathy might be present in hypothyroidism and associated with Wernicke-Korsakoff syndrome.
Numbness or sensory loss may be noted in stroke patients.
Patients with vitamin B12 deficiency might have predominant posterior column signs of decreased vibration and proprioception in addition to their cognitive complaints. Lower extremity numbness may also be seen.
Cortical sensory deficits (e.g., astereognosis, agraphesthesia, extinction to double simultaneous stimuli) are seen with corticobasal degeneration, corticobasal syndrome, and advanced AD.
Reflexes
Frontal release signs (e.g., grasp, palmomentalis, rooting) are completely nonspecific for any particular disorder but are seen in general encephalopathies; only the grasp has localizing value to the frontal lobes.
Delayed relaxation of deep tendon reflexes are observed in hypothyroidism.
Heightened tendon reflexes, grasp/suck reflexes, and a positive Babinski sign might be seen in HIV dementia.
Hyporeflexia and a positive Babinski sign might be seen in vitamin B12 deficiency.
Unilateral hyperreflexia and a positive Babinski sign might occur in a stroke.
Gait
Ataxia might be seen in many dementias, including CJD, HIV dementia, limbic encephalopathy (and various autoimmune paraneoplastic disorders), Hashimoto encephalopathy, ischemic stroke/vascular dementia, vitamin B12 deficiency, and Wernicke-Korsakoff syndrome.
Bradykinetic gait with stooped posture and decreased arm swing is seen among the Parkinson spectrum disorders (corticobasal syndrome [usually due to corticobasal degeneration, PSP, or Alzheimer disease], PSP, and dementia with Lewy bodies). These patients are particularly susceptible to retropulsion testing.
Magnetic gait is often seen with normal pressure hydrocephalus.
Hemiparetic gait may be found in vascular dementia.
Large fiber sensory loss with positive Romberg sign (due to posterior column degeneration) might be present in neurosyphilis.
Other
Dry skin, pretibial myxedema, and hearing impairment might be present in hypothyroidism.
Delayed speech output with long pauses between words may occur in HIV dementia.
Neuropsychological testing
The third part of the workup is neuropsychological testing. In many cases, a neuropsychological evaluation helps define the pattern and extent of deficits.[56]
Memory
Significant short-term memory deficits (e.g., trouble recalling lists of words or reconstructing a two-dimensional figure from memory) are seen on neuropsychological testing in AD. Episodic memory is also affected in limbic encephalopathy and Wernicke-Korsakoff encephalopathy.
Acute onset of episodic memory deficits lasting on average 4 to 6 hours in the absence of other neurologic symptoms, or retrograde amnesia extending as far back as weeks or months, might be present in transient global amnesia.
Episodic memory loss may be present with hypoxic injury secondary to cardiac arrest. This is most severe post-arrest, and often improves quickly over days. There also might be chronic impairment in working and episodic memory.
Anterior thalamic infarcts may result in episodic memory impairment.
Semantic memory is particularly affected in semantic dementia, whereas episodic memory is usually preserved until later stages of the disease.
Frontal pattern of memory loss with impaired retrieval but intact encoding is commonly observed in vascular dementia, frontotemporal dementia, other frontal dementias (e.g., PSP), as well as normal aging.
Frontal-subcortical dementia (e.g., forgetfulness; bradyphrenia; emotional/personality changes such as apathy, depression, and irritability; inability to manipulate acquired knowledge) occurs in frontotemporal dementia, PSP, and corticobasal degeneration. Deficits in spontaneous recall with preserved recognition are also seen on memory testing. Patients with corticobasal degeneration have also been found to have problems with episodic, semantic, and working memory.
Confabulation (false memories) is often seen in Wernicke-Korsakoff syndrome.
Patients with temporal lobe epilepsy might suffer from chronic deficits of encoding, storage, and retrieval of new information, in addition to memory loss during the seizure.
Executive
Frontal executive deficits (e.g., poor concentration, disorganization, problems multi-tasking) may occur in vascular dementia, frontotemporal dementias, HIV dementia, normal pressure hydrocephalus, and hypoxic injury after cardiac arrest.
Certain thalamic infarcts might lead to executive dysfunction.
Visuospatial
Visuospatial deficits are common across many differential diagnoses including AD, dementia with Lewy bodies, CJD, and strokes.
Acalculia (inability to perform simple mathematic tasks) may occur in AD, corticobasal syndrome, or CJD.
A typical brief bedside battery of neuropsychological testing might include the following:[57]
Mini Mental State Examination (MMSE)
This is a brief measure that tests six areas of cognitive function: orientation, registration, attention, recall, language, and visuospatial function. The maximum score is 30. Some regard a score of less than 24 as indicative of dementia.
Although an effective screening tool, it relies heavily on verbal response and reading and writing and does not assess frontal-executive function well.
When used serially over time, the MMSE is able to measure changes in cognitive status.
Those with hearing or visual impairment or low English literacy might perform poorly even when cognitively intact.
There is also a short form of the MMSE (SMMSE) that consists of the six memory items of the MMSE (immediate recall of three words and delayed recall of the three words). The score range is 0 to 6, with 6 as the best performance and the cutoff score for dementia being less than 4. One study showed that the SMMSE has the same sensitivity and higher specificity than MMSE for screening dementia among community-dwelling older adults. Another study showed that the SMMSE is a good screening test for following up with older individuals in a memory clinic.[58][59]
Montreal Cognitive Assessment (MoCA)
Similar to the MMSE, this is a 30-point test and, like the MMSE, it assesses orientation, attention (registration [not scored], target detection using tapping), short-term verbal memory, visuospatial abilities (clock-drawing; three-dimensional cube copy), and language (a three-item confrontation naming, repetition of two complex sentences, a phonemic fluency task).
Unlike the MMSE, it also assesses executive functions (an alternation task adapted from the trail-making B task, a phonemic fluency task, a two-item verbal abstraction) and working memory (target detection using tapping, a serial subtraction task, digits forward and backward).[60]
A score of 26 or above generally is considered normal. Montreal Cognitive Test Opens in new window
A few other significant advantages of this test over the MMSE is that it is available and validated in dozens of languages and is not proprietary. The test takes ≤10 minutes to complete.
Abbreviated Mental Test Score (AMTS)
Consists of 10 questions and each correctly answered question scores one point. Systematic review and meta-analysis of two UK studies and one Italian study using the AMTS as a screening instrument for dementia in hospital inpatients older than 60 years of age showed that the AMTS has a sensitivity of 81% and a specificity of 84%, with a cut-off value of <7 in diagnosing dementia.[61] The test takes ≤10 minutes to complete.
Mini-Cog
A simple test that includes three-word recall and clock drawing. A recall score of 0, or a recall score of 1 to 2 plus abnormal clock drawing, are considered positive for cognitive impairment. The test takes ≤5 minutes to complete.
Addenbrooke's Cognitive Examination-Revised (ACE-R)
A brief battery that examines orientation, attention, memory, verbal fluency, language, and visuospatial ability. A total score is 100, with a cut-off score of 82 (sensitivity 84% and specificity 100% for dementia). The test takes ≤20 minutes to complete.
Other brief, multi-domain screening tests[48] [ Cognitive Impairment Screening with 6 Questions Opens in new window ]
10-point cognitive screener
Six-item cognitive impairment test (6CIT)
Six-item screener
Memory impairment screen (MIS)
Test Your Memory (TYM).
International HIV Dementia Scale (IHDS)
May be used to rapidly screen patients with suspected HIV dementia. The IHDS consists of three subtests: timed finger tapping, timed alternating hand sequence test, and recall of four items at 2 minutes. A score of ≤10 is an indication for further neuropsychological evaluation.[62]
One Cochrane review of the MMSE for the detection of dementia in community and primary care populations concluded that the MMSE contributes to a diagnosis of dementia, but should not be used in isolation to confirm or exclude disease.[63] Years of education predict higher false-negative rates for diagnosing dementia using the MMSE, whereas nursing home residency predicts higher false-positive diagnoses.[64]
One systematic review and meta-analysis of 149 studies of cognitive tests for detecting dementia found that the Mini-Cog and ACE-R tests were comparable to the MMSE for detecting dementia, while MoCA was comparable to the MMSE for detecting mild cognitive impairment.[65] However, one Cochrane review found that there is insufficient evidence to recommend the Mini-Cog as a screening tool for dementia in the primary care setting.[66]
California Verbal Learning Test (CVLT) or Hopkins Verbal Learning Test (HVLT)
The CVLT and HVLT assess verbal memory abilities. Briefly, the tester reads aloud a list of common words (9, 12, or 16), each of which belongs to one of three or four categories (e.g., fruit, clothing, tools). This is repeated three to five times. After the list is read by the examiner, each time the subject is asked to repeat back as many of the items as possible. After a short delay (~30 seconds), the subject is again asked to recall the word list (in more challenging versions, the tester might give a second list, and see if the subject is able to keep the items from each list separate, or if the two lists become confused). Finally, there is a long delay (10 or 20 minutes), during which the subject is given other tasks to perform, and then the subject is asked to recall the (first) word list spontaneously, then with cues and then a yes/no recognition task.
This test measures verbal episodic short-term memory, whether or not the subject is making use of category information (executive function), and if helped with retrieval (e.g., cues or recognition), which are frontal lobe tasks.
Immediate free recall tests can be useful for predicting progression of patients with memory complaints to Alzheimer disease over the next few years, although some types of memory tests have better predictive value than others.[67]
Modified or mini-Boston Naming Test (BNT)
The BNT is a 60-item confrontation naming test (picture-naming vocabulary test) and measures the word retrieval performance. The 60 items consist of high-frequency and low-frequency words. The patients with dysnomia often have greater difficulties with the naming of low-frequency objects.
Visuospatial testing (e.g., cube and clock drawing; both in MoCA)
Visuospatial memory can be tested by asking the subject to copy a cube (as in MoCA) or to reproduce a more complex line drawing such as the Rey-Osterreith figure or the simplified version, the Benson figure, first by copying and then several minutes later drawing it from memory.
Visual memory test (recall of a figure drawn earlier)
Another simple memory task for patients who are too impaired for the CVLT, HVLT, or complex figure memory is to hide three bills around the room. Several minutes later you ask what was hidden (verbal memory) and where (visual memory).
Tests of executive function include:
Verbal fluency (semantic and phonemic or lexical fluency) - number of words beginning with certain letter(s) in one minute and number of animals or groceries in one minute.
Design fluency test (making a different design by connecting dots with straight lines).
Trail making test part B (drawing lines alternating between numbers and letters in ascending order).
Stroop test. The Stroop test takes advantage of our ability to read words more quickly and automatically than we can name colors. When a word is printed or displayed in a color different from the color it actually names, the subject is asked to respond with the color ink, and inhibit (disregard) the word you read (e.g., the word "green" in blue ink should be read as “blue”). The cognitive mechanism involved in this task is called “directed attention” and requires managing attention, inhibiting an innate response in order to say or do something else. It measures mental (attentional) vitality and flexibility.
Simple calculations. Calculations can be impaired by executive difficulties (not carrying-over appropriately). Calculations can be tested by doing simple one- and/or two-digit addition, subtraction, division, and multiplication. One can start with a hard multiplication task, such as 214 x 35; if the subject gets this correct, one can skip simpler calculations.
Neuroimaging
The next step of the diagnostic workup is neuroimaging. A structural imaging study is recommended for the workup of dementia, and likewise should be performed in a patient with memory loss.[48][68]
The most appropriate study for any patients with memory loss is a brain magnetic resonance imaging (MRI). MRI with T2, diffusion-weighted imaging, and gradient echo sequencing is the preferred method over computed tomography (CT) scan. MRIs are preferred because they have better resolution, differentiation of gray-white matter borders, and sequences for identification of vascular disease.
Neuroimaging serves to distinguish a neurodegenerative disease from other processes such as vascular lesions, mass lesions, infectious etiologies, and inflammatory processes. A brain MRI should be done in all patients with memory loss, possibly even if patients meet all the clinical criteria for Alzheimer disease, in order to:
Rule out other possible causes, particularly reversible, of dementia, such as structural lesions
Examine the presence of a comorbid pathology
Get a baseline imaging before memory loss progresses further.
Fluid-attenuated inversion-recovery imaging of coronal thin sections through the temporal lobes can help with detecting early hippocampal sclerosis and atrophy. [Figure caption and citation for the preceding image starts]: MRI brain: shows diffuse cortical atrophy with predominant hippocampal atrophy (red arrows) in patient with Alzheimer dementia. Parietal atrophy and neocortical involvement is evident in more advanced disease (blue arrows)From the personal collection of Michael D. Geschwind, MD, PhD; used with permission [Citation ends].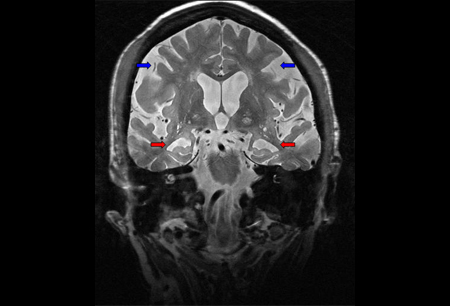 The structural image typically shows a corresponding atrophy pattern to the patient's clinical deficits. Patients with memory loss frequently have MRI findings involving the medial temporal lobes (hippocampi). Lesions to other regions of Papez's circuit, including the anterior thalamus and the mammillary bodies, may also be found in a patient with memory loss.
The structural image typically shows a corresponding atrophy pattern to the patient's clinical deficits. Patients with memory loss frequently have MRI findings involving the medial temporal lobes (hippocampi). Lesions to other regions of Papez's circuit, including the anterior thalamus and the mammillary bodies, may also be found in a patient with memory loss.
CT and MR angiography are indicated when ischemic stroke or vasculitis is a suspected cause of memory loss. MR angiography can be obtained at the same time as MRI.[29] Head CTs are better for imaging bleeds.[69] Although MRI is more sensitive than CT for acute infarcts, CT is often obtained first to assess for hemorrhage or large infarct. This allows the treating clinician to rapidly make decisions about thrombolysis.[29] Noncontrast head CT is the investigation of choice for a traumatic brain injury when imaging is needed.[70]
Functional imaging with fluorodeoxyglucose positron emission tomography (FDG-PET) scan and CT/single-photon emission computed tomography (CT-SPECT) might be used to differentiate AD from frontotemporal dementia. Frontotemporal dementia has frontal hypometabolism, whereas AD has more posterior temporoparietal hypoperfusion.[48][68] In the case of frontal variant AD, however, it may not be as helpful. One study comparing cohorts of probable AD patients versus probable dementia with Lewy bodies patients found that, on average, patients with dementia with Lewy bodies had more occipital hypoperfusion on perfusion SPECT, and smaller putamen on volumetric MRI, than the AD cohort.[71] As none of these cases were pathology-confirmed, tested for amyloid imaging, or had cerebrospinal fluid (CSF) biomarkers for AD diagnosis, it is not yet clear whether these techniques are any better than standard clinical diagnosis.
Laboratory investigations
Initial investigations
The likelihood of identifying a reversible cause of dementia is estimated to be about 9%.[3] In general, initial laboratory studies that should be ordered in all patients to identify potentially reversible causes of memory loss include:
Serum electrolytes (including calcium, magnesium and phosphorus)
Blood urea nitrogen/renal function
Thyroid function tests
Serum vitamin B12
Complete blood count
Erythrocyte sedimentation rate/C-reactive protein.
Thiamine levels may also be appropriate. When vascular dementia is suspected, a lipid panel and fasting glucose or hemoglobin A1c (HbA1c) measurement are important for the treatment of risk factors. The role of 25-hydroxyvitamin D deficiency in cognitive impairment is controversial; although lower levels are more associated with dementia, causality has not been determined.[72][73][74] Measuring serum vitamin D, therefore, currently is not yet recommended as part of a standard workup.
Investigations for infectious causes
Although the rapid plasma reagin test for syphilis is not recommended routinely, it should be performed if the patient has a specific risk factor or there are other clinical features of neurosyphilis.[68] Many physicians order this test because syphilis is a reversible form of dementia that often goes undiagnosed. This may be particularly important in regions with a higher prevalence of syphilis.
HIV serology should also be ordered in any patient with risk factors or suspected infectious etiology to their dementia.
Investigations for inflammatory causes
If a limbic encephalopathy is present, serum (and sometimes CSF) should be sent for paraneoplastic and nonparaneoplastic antibodies (e.g., anti-Hu, Ma2, CV2, voltage-gated potassium channel-associated, N-methyl D-aspartate receptor, thyroglobulin antibodies [anti-TG], thyroid peroxidase [anti-TPO] and several other antibodies).
When testing for antibody-mediated limbic encephalopathy, including paraneoplastic disease, for several reasons it is recommended to send complete panels. Many patients have more than one antibody. Some antibodies are associated with certain syndromes, and some antibodies are associated with certain cancers.[34][75][76]
Genetic testing
Genetic testing is indicated when there is a question of inherited forms of dementia. This is often a consideration in early-onset AD, frontotemporal dementia, and Creutzfeldt-Jakob disease. Patients who carry the APOE4 genotype are more susceptible to developing dementia, but typically present later in life. Patients with mutations in presenilin-1, presenilin-2, and amyloid precursor protein tend to present with early-onset AD.
Some genetic mutations associated with frontotemporal dementia include progranulin, microtubule-associated protein tau, and C9ORF72.[77] C9ORF72 hexanucleotide repeat expansion (>30 repeats) is the most common mutation identified in frontotemporal dementia patients, and has been seen most commonly in patients from Europe and the US, but rarely in those from Asia. Clinically, it manifests most commonly as behavioral variant frontotemporal dementia, followed by primary progressive aphasia. This mutation has also been identified in patients diagnosed with AD, Huntington disease-like syndromes, and Parkinson disease. The extent of this mutation is still to be determined.[78][79] One study at a frontotemporal dementia referral center found that 12% of patients had the C9ORF72 mutation. Carriers of the mutation were much more likely to have a family history of amyotrophic lateral sclerosis or psychiatric illness (hallucinations and delusions). Carriers also had slower progression and less prominent brain atrophy than those without the mutation.[80] The pathologic mechanism of the C9ORF72 repeat mutation-induced neurodegeneration appears to be due to RNA aggregates, and is an intense area of current research.[77][81][82][83][84]
Whenever genetic testing is performed, genetic counseling is essential. Guidelines for performing genetic testing in Huntington disease (HD) and other autosomal dominant neurodegenerative dementias are continually being rewritten based on new technologies, but are based on the original guidelines for HD, which are often referred to as the Huntington Protocol.[85]
Urine tests
If there is clinical suspicion of a urinary tract infection, send a midstream urine sample for microscopy and culture.[48] Urinary tract infections may precipitate delirium, which manifests as a sudden cognitive decline.
Cerebrospinal fluid (CSF) investigations
CSF studies should be done in atypical presentations of dementia in which infectious, autoimmune, inflammatory, Creutzfeldt-Jakob disease, and neoplastic causes are considerations.
CSF tau levels are elevated and amyloid beta 1-42 (Abeta42) levels are decreased in AD. CSF testing for Abeta42, total-tau, and phosphorylated tau ratios have sensitivity and specificity in the mid 85th percentile range. These biomarker tests appear to have better sensitivity than specificity and may have greater use in ruling out Alzheimer disease as an etiology for the individual's cognitive impairment, as opposed to confirming it.[86]
When considering normal pressure hydrocephalus, a large volume lumbar puncture (at least 30 mL) or prolonged CSF drainage might be performed to assess whether symptoms, especially gait, improve following the lumbar puncture.[87][88]
If limbic encephalopathy is present, CSF may be sent for paraneoplastic and nonparaneoplastic antibodies.
Other investigations
Electroencephalogram is essential to rule out subclinical seizures when seizures are suggested by the clinical presentation. In such cases, patients might have other symptoms in addition to memory loss, such as staring spells, sudden halting of speech, or subtle facial, lip, or eye twitching. For more acute declines in mental status without other explanation, nonconvulsive status epilepticus should be considered, in which there are no motor manifestations of seizures.[31]
Cognitive impairment: recognition, diagnosis and management in primary care Opens in new window
Use of this content is subject to our disclaimer