Approach
Rocky Mountain spotted fever (RMSF) is a notifiable disease in the US. Clinicians should prescribe antibiotic therapy (doxycycline) once the diagnosis is reasonably considered, without waiting for results of confirmatory tests. Early consideration of the diagnosis and initiation of therapy is important as the case fatality rate increases considerably after 5 days of illness. This presents a challenge, as no constellation of signs and symptoms definitely confirms or excludes the diagnosis, and fever with headache is a common presentation of many illnesses.
History
During the spring and summer in endemic areas, RMSF should be seriously considered in any patient with fever and headache, regardless of the tick exposure history. A detailed history of recent recreational or occupational outdoor activities may reveal potential tick exposures that were unknown to the patient. A history of tick bite may not be elicited in up to 45% of cases. An inoculation eschar at the site of the tick bite is rarely present.[1]
Clinical presentation
In most cases, clinical features begin 3 to 12 days after the bite attachment of an infected tick. Because RMSF is a multisystem vasculitis, its manifestations can be related to virtually any organ. Approximately two-thirds of patients present with fever, rash, and headache, and almost all patients will have fever with headache.[1][14][15][18] Myalgia and malaise are usually present. Other reported clinical features include nausea, vomiting, abdominal pain and diarrhea.
Initial symptoms are vague and nonspecific, and the rash is only rarely present in the first 3 days of illness in adults. It can, however, manifest earlier in the disease course in children. The rash typically begins as small (1-5 mm), blanching erythematous macules on the ankles and wrists which then spreads proximally to the arms and legs, and palms and soles before involving the trunk. The face is usually spared. Over a few days the rash becomes maculopapular and subsequently petechial. Purpura fulminans is a late finding of severe infection. Classic petechiae usually appear by day 5 or 6 and are associated with severe disease. It should be noted that the rash may be absent altogether in a small proportion of adults and children with RMSF. The presence of a maculopapular or petechial rash markedly increases the likelihood of RMSF; however, the absence of a rash does not exclude the diagnosis.[1][Figure caption and citation for the preceding image starts]: Child's right hand and wrist displaying the characteristic spotted rash of Rocky Mountain spotted feverCDC Image Library; used with permission [Citation ends].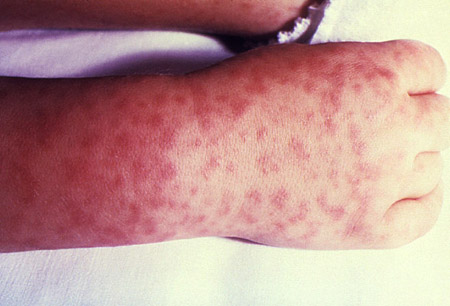
Less frequently noted findings that may occur later in the presentation include mental status impairment, meningismus/meningitis, conjunctivitis, lymphadenopathy, periorbital or peripheral edema, hepatosplenomegaly, or jaundice. Abdominal pain mimicking appendicitis, or gastroenteritis, may be observed and is more common in children. Uncommon findings include pneumonitis, focal neurologic deficits, coma, seizures, shock, arrhythmias, or myocarditis.
It is important to note that the clinical presentation of cases in Arizona may differ from the usual presentation in the US.[6]
CDC: timeline of RMSF signs and symptoms Opens in new window
Laboratory investigations
CBC, electrolytes, LFTs, and blood culture should be evaluated in patients with suspected RMSF. Hyponatremia, thrombocytopenia, and mildly elevated AST and ALT are all suggestive of RMSF; however, absence of any of these does not exclude the diagnosis.[1][19]
Blood culture should be performed, because meningococcemia (or, rarely, bloodstream infections with other pyogenic bacteria) can present with similar signs and symptoms.
Cerebrospinal fluid (CSF) examination is not necessary in most cases of suspected RMSF, but may be performed as part of the diagnostic evaluation of patients with fever and abnormal sensorium or other neurologic findings. The CSF of patients with RMSF typically demonstrates mononuclear cell pleocytosis (<100 cells/microliter), elevated protein concentration, and normal glucose concentration.
Findings of disseminated intravascular coagulation are rare.
Chest and abdominal imaging may suggest pulmonary edema, systemic vasculitis, or can be useful for excluding other diagnoses such as appendicitis.
Serology
Patients with RMSF do not usually demonstrate a serologic response against Rickettsia rickettsii until at least 7 to 10 days into the course of illness. Thus, serologic testing is done for confirmation purposes only; clinicians should prescribe therapy as soon as the diagnosis is reasonably considered, without waiting for the results of confirmatory tests.[1][19]
Indirect immunofluorescent antibody (IFA) is the preferred serologic test and IFA from paired acute and convalescent serum is the reference standard; other options include enzyme immunoassay, complement fixation (CF), latex agglutination (LA), indirect hemagglutination (IHA), and microagglutination (MA) tests.
If the single serum titer is ≥1:64 by IFA, ≥1:16 by CF, or ≥1:128 by LA, IHA, or MA in a patient with a compatible clinical illness, there is a probable diagnosis of RMSF. If results are negative in a patient in the first week of illness, serology should be repeated. Early therapy with doxycycline might impair development of RMSF antibodies.
Confirmation of the diagnosis requires a 4-fold or greater change in titer between acute-phase and convalescent-phase serum specimens. The Weil-Felix serologic test, which measures responses to Proteus vulgaris OX-19 and OX-2 agglutinins, is inferior to currently available specific antirickettsial serologic tests and is no longer recommended.[1][13]
Serologic diagnosis does not differentiate between spotted fever group Rickettsia species in most laboratories.[1][20]
Other investigations
Immunohistochemistry can confirm the diagnosis early in the illness course by demonstrating the presence of rickettsiae in skin biopsy samples, but it is not commonly used. This technique has a reported diagnostic sensitivity of about 70% and specificity of 100% but is time consuming and available only in specialized research laboratories and the CDC.[1][19]
Polymerase chain reaction can confirm the diagnosis early in the illness course by demonstrating the presence of Rickettsia rickettsii DNA in blood or biopsy specimens, but it is not commonly used. This technique is not adequately sensitive to exclude the diagnosis due to low numbers of rickettsia in circulating blood in patients with less severe disease. It is currently available only in specialized research laboratories and at the CDC.[1][19]
CDC: rickettsial disease diagnostic testing and interpretation Opens in new window
Use of this content is subject to our disclaimer