Criteria
Beckman classification of AMD[2]
No AMD
No or a few small (<63 micrometers in diameter) drusen.
Early AMD[Figure caption and citation for the preceding image starts]: Early AMD (Age-Related Eye Disease Study Group [AREDS] category 2)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].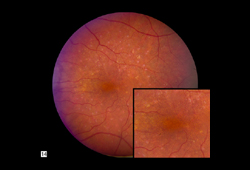
Intermediate-sized (63-124 micrometers in diameter) drusen.
Intermediate AMD[Figure caption and citation for the preceding image starts]: Intermediate AMD (Age-Related Eye Disease Study Group [AREDS] category 3)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].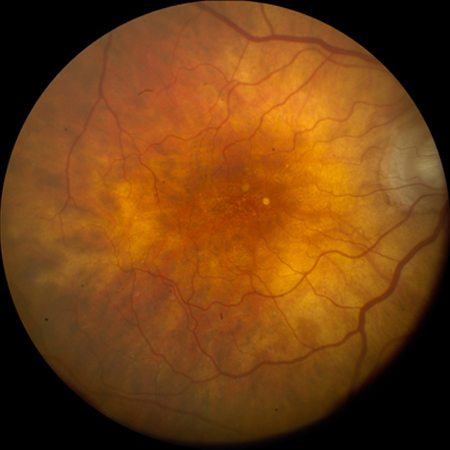
Intermediate drusen and pigmentary changes, or at least one large (≥125 micrometers) druse.
Late AMD
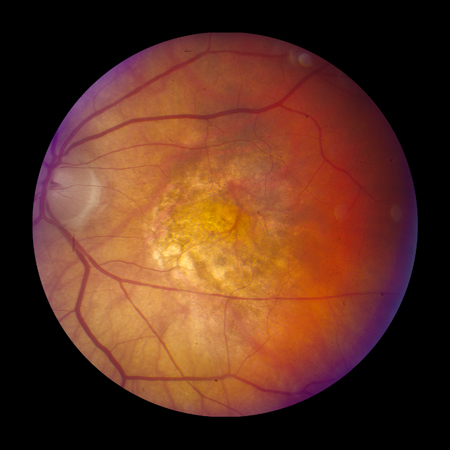
Geographic atrophy[Figure caption and citation for the preceding image starts]: Late AMD with central geographic atrophy (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
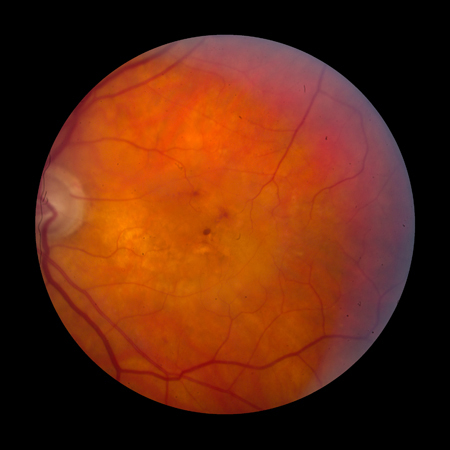
Choroidal neovascularization (CNV) with signs including subretinal hemorrhage, serous retinal or retinal pigment epithelium detachments, lipid exudates, or fibrovascular scar.[Figure caption and citation for the preceding image starts]: Late AMD with choroidal neovascularization with exudation (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Fibrovascular scar from end-stage AMD (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Fibrovascular scar from end-stage AMD (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].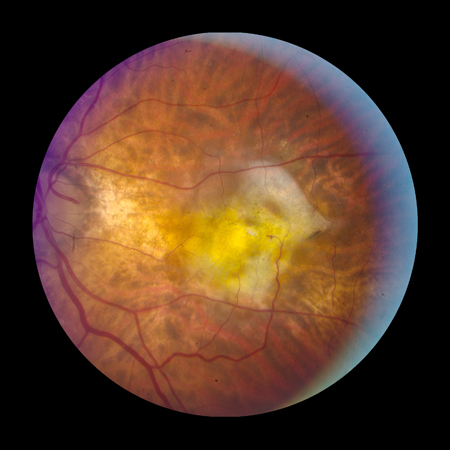
Age-Related Eye Disease Study Group (AREDS) classification[23]
The AREDS study investigated the use of antioxidants and mineral supplementation to decrease the risk of AMD progression. In this study, AMD was classified as follows:[34]
No AMD (AREDS category 1)
No or a few small (<63 micrometers in diameter) drusen.
Early AMD (AREDS category 2)[Figure caption and citation for the preceding image starts]: Early AMD (Age-Related Eye Disease Study Group [AREDS] category 2)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
Many small drusen, or a few intermediate-sized (63-124 micrometers in diameter) drusen, or mild abnormalities of the retinal pigment epithelium (RPE).
Intermediate AMD (AREDS category 3)[Figure caption and citation for the preceding image starts]: Intermediate AMD (Age-Related Eye Disease Study Group [AREDS] category 3)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
Extensive intermediate drusen, or at least one large (≥125 micrometers in diameter) druse, or geographic atrophy not involving the foveal center.
Advanced AMD (AREDS category 4): one or more of the following in one eye (in the absence of other causes):
Geographic atrophy involving the foveal center (atrophic, or dry, AMD)[Figure caption and citation for the preceding image starts]: Late AMD with central geographic atrophy (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].

Neovascular maculopathy (exudative, wet AMD), including CNV, serous and/or hemorrhagic detachment of the retina or RPE, retinal hard exudates, subretinal and sub-RPE fibrovascular proliferation, or disciform scar (subretinal fibrosis).[Figure caption and citation for the preceding image starts]: Late AMD with choroidal neovascularization with exudation (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Fibrovascular scar from end-stage AMD (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Fibrovascular scar from end-stage AMD (Age-Related Eye Disease Study Group [AREDS] category 4)Reproduced from Scheie Eye Institute's patient image database; used with permission [Citation ends].
Use of this content is subject to our disclaimer