The diagnosis of Wernicke encephalopathy is challenging and often missed, with the majority of cases identified on postmortem exam.[16]Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986 Apr;49(4):341-5.
http://www.ncbi.nlm.nih.gov/pubmed/3701343?tool=bestpractice.com
[18]Torvik A, Lindboe CF, Rogde S. Brain lesions in alcoholics: a neuropathological study with clinical correlations. J Neurol Sci.1982 Nov;56(2-3):233-48.
http://www.ncbi.nlm.nih.gov/pubmed/7175549?tool=bestpractice.com
[26]Kohnke S, Meek CL. Don't seek, don't find: the diagnostic challenge of Wernicke's encephalopathy. Ann Clin Biochem. 2021 Jan;58(1):38-46.
https://journals.sagepub.com/doi/10.1177/0004563220939604
http://www.ncbi.nlm.nih.gov/pubmed/32551830?tool=bestpractice.com
The classic clinical triad of mental status changes, ophthalmoplegia, and gait dysfunction may only be present in up to 16% of patients with Wernicke encephalopathy; around 19% of patients may present without any classical signs.[16]Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986 Apr;49(4):341-5.
http://www.ncbi.nlm.nih.gov/pubmed/3701343?tool=bestpractice.com
Given the clinical variability of the condition, a high index of suspicion is required when assessing patients. Treatment should not be delayed pending the results of investigations, owing to the risk of permanent neurologic injury and death.[1]Galvin R, Bråthen G, Ivashynka A, et al. Guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010 Dec;17(12):1408-18.
http://onlinelibrary.wiley.com/doi/10.1111/j.1468-1331.2010.03153.x/full
http://www.ncbi.nlm.nih.gov/pubmed/20642790?tool=bestpractice.com
History
A history of alcohol-use disorder, poor/unbalanced dietary intake, vomiting, diarrhea, fever, coexisting medical conditions (e.g., cancer or kidney disease), immunodeficiency, or gastrointestinal surgery should be elicited. Thiamine deficiency presenting as Wernicke encephalopathy is often linked with alcohol-use disorders, particularly in developing countries, however other potential causes of the condition should be thoroughly explored.[1]Galvin R, Bråthen G, Ivashynka A, et al. Guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010 Dec;17(12):1408-18.
http://onlinelibrary.wiley.com/doi/10.1111/j.1468-1331.2010.03153.x/full
http://www.ncbi.nlm.nih.gov/pubmed/20642790?tool=bestpractice.com
[69]Cantu-Weinstein A, Branning R, Alamir M, et al. Diagnosis and treatment of Wernicke's encephalopathy: a systematic literature review. Gen Hosp Psychiatry. 2024 Mar-Apr;87:48-59.
http://www.ncbi.nlm.nih.gov/pubmed/38306946?tool=bestpractice.com
In one series of 53 case reports, 81% of Wernicke encephalopathy cases were not alcohol-related.[70]Lough ME. Wernicke's encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev. 2012 Jun;22(2):181-94.
http://www.ncbi.nlm.nih.gov/pubmed/22577001?tool=bestpractice.com
Exam
The classic clinical triad of mental status changes, ophthalmoplegia, and gait dysfunction is only present in up to 16% of cases; therefore, a thorough exam is required in patients with suspected Wernicke encephalopathy.[16]Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986 Apr;49(4):341-5.
http://www.ncbi.nlm.nih.gov/pubmed/3701343?tool=bestpractice.com
Exam should be focused and cover assessment of mental status, cranial nerve function (especially the optic and oculomotor nerves), vestibular and gait function, strength, reflexes, and fundoscopy.
It is important to note that while the classic triad describes ophthalmoplegia, nystagmus is a more common finding.[71]Isen DR, Kline LB. Neuro-ophthalmic manifestations of Wernicke encephalopathy. Eye Brain. 2020;12:49-60.
https://www.dovepress.com/neuro-ophthalmic-manifestations-of-wernicke-encephalopathy-peer-reviewed-fulltext-article-EB
http://www.ncbi.nlm.nih.gov/pubmed/32636690?tool=bestpractice.com
Other oculomotor findings include gaze palsies, sixth nerve palsies, and impaired horizontal vestibulo-ocular reflexes. Patients may also have miosis, anisocoria, light/near dissociation, papilledema, and retinal hemorrhages.
Mental status changes that may be evident include inattentiveness, apathy, a decreased level of consciousness, and memory impairment.[71]Isen DR, Kline LB. Neuro-ophthalmic manifestations of Wernicke encephalopathy. Eye Brain. 2020;12:49-60.
https://www.dovepress.com/neuro-ophthalmic-manifestations-of-wernicke-encephalopathy-peer-reviewed-fulltext-article-EB
http://www.ncbi.nlm.nih.gov/pubmed/32636690?tool=bestpractice.com
Ataxia presents mainly as gait dysfunction due to loss of equilibrium. Patients can develop a wide-based gait and struggle to walk, or be unable to stand.[71]Isen DR, Kline LB. Neuro-ophthalmic manifestations of Wernicke encephalopathy. Eye Brain. 2020;12:49-60.
https://www.dovepress.com/neuro-ophthalmic-manifestations-of-wernicke-encephalopathy-peer-reviewed-fulltext-article-EB
http://www.ncbi.nlm.nih.gov/pubmed/32636690?tool=bestpractice.com
Retrospective data suggest that patients with alcohol-use disorder and Wernicke encephalopathy are more likely to present with cerebellar signs, and less likely to present with oculomotor signs than patients with nonalcohol-related Wernicke encephalopathy. In this study, diagnosis was delayed in patients without alcohol-use disorder compared with patients who had the disorder (median time to diagnosis: 4 vs. 1 day/s).[72]Chamorro AJ, Rosón-Hernández B, Medina-García JA, et al; Wernicke-SEMI Group; Alcohol and Alcoholism Group; Spanish Society of Internal Medicine (SEMI). Differences between alcoholic and nonalcoholic patients with Wernicke encephalopathy: a multicenter observational study. Mayo Clin Proc. 2017 Jun;92(6):899-907.
http://www.ncbi.nlm.nih.gov/pubmed/28578781?tool=bestpractice.com
More uncommonly, patients may develop hearing loss, epileptic seizures, hypothermia or hyperthermia, spastic paraparesis, and tachycardia or hypotension related to the cardiovascular manifestations of thiamine deficiency.[23]Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007 May;6(5):442-55.
http://www.ncbi.nlm.nih.gov/pubmed/17434099?tool=bestpractice.com
[73]Ota Y, Capizzano AA, Moritani T, et al. Comprehensive review of Wernicke encephalopathy: pathophysiology, clinical symptoms and imaging findings. Jpn J Radiol. 2020 Sep;38(9):809-20.
https://link.springer.com/article/10.1007/s11604-020-00989-3
http://www.ncbi.nlm.nih.gov/pubmed/32390125?tool=bestpractice.com
Investigations
The diagnostic challenge in Wernicke encephalopathy generally lies in the lack of sufficiently sensitive and specific diagnostic tests. Wernicke encephalopathy remains a clinical diagnosis; therefore, treatment should not be delayed pending the results of investigations. A positive diagnosis is often considered established if there is a manifest reversal of clinical signs with parenteral administration of thiamine.[26]Kohnke S, Meek CL. Don't seek, don't find: the diagnostic challenge of Wernicke's encephalopathy. Ann Clin Biochem. 2021 Jan;58(1):38-46.
https://journals.sagepub.com/doi/10.1177/0004563220939604
http://www.ncbi.nlm.nih.gov/pubmed/32551830?tool=bestpractice.com
Laboratory
A comprehensive blood panel should be ordered to include serum thiamine, blood alcohol level, blood glucose, serum magnesium, a complete blood count, serum electrolytes, renal and liver function tests, and serum ammonia. Urine and blood toxicology screens should also be ordered in all patients presenting with suspected of Wernicke encephalopathy. A lumbar puncture may be performed if there is a clinical suspicion of encephalitis, meningitis, or subarachnoid hemorrhage.
Where possible, serum thiamine should be measured prior to treatment with parenteral administration of thiamine, however this should not delay its administration.[1]Galvin R, Bråthen G, Ivashynka A, et al. Guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010 Dec;17(12):1408-18.
http://onlinelibrary.wiley.com/doi/10.1111/j.1468-1331.2010.03153.x/full
http://www.ncbi.nlm.nih.gov/pubmed/20642790?tool=bestpractice.com
A normal thiamine level does not always exclude the condition as up to 8% of patients with Wernicke encephalopathy may have a normal or high thiamine level.[70]Lough ME. Wernicke's encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev. 2012 Jun;22(2):181-94.
http://www.ncbi.nlm.nih.gov/pubmed/22577001?tool=bestpractice.com
In rare cases, this may be explained by mutations in thiamine metabolism and transport genes.
Imaging
Magnetic resonance imaging (MRI) is the preferred imaging modality in the workup of Wernicke encephalopathy and has been shown to have a sensitivity of 53% and a specificity of 93%.[1]Galvin R, Bråthen G, Ivashynka A, et al. Guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010 Dec;17(12):1408-18.
http://onlinelibrary.wiley.com/doi/10.1111/j.1468-1331.2010.03153.x/full
http://www.ncbi.nlm.nih.gov/pubmed/20642790?tool=bestpractice.com
[74]Antunez E, Estruch R, Cardenal C, et al. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol. 1998 Oct;171(4):1131-7.
https://www.ajronline.org/doi/10.2214/ajr.171.4.9763009
http://www.ncbi.nlm.nih.gov/pubmed/9763009?tool=bestpractice.com
[75]Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke's encephalopathy and Korsakoff's syndrome. Neuropsychol Rev. 2012 Jun;22(2):170-80.
http://www.ncbi.nlm.nih.gov/pubmed/22577003?tool=bestpractice.com
[76]Kitaguchi T, Ota Y, Liao E, et al. The role of MRI in the prognosis of Wernicke's encephalopathy. J Neuroimaging. 2023 Nov-Dec;33(6):917-25.
https://onlinelibrary.wiley.com/doi/10.1111/jon.13143
http://www.ncbi.nlm.nih.gov/pubmed/37355834?tool=bestpractice.com
Although both MRI and computed tomography (CT) have low sensitivity in this context, MRI is seen as more advantageous when compared to CT given it can visualize and quantify edematous lesions rather than only detecting them as low-density abnormalities.[75]Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke's encephalopathy and Korsakoff's syndrome. Neuropsychol Rev. 2012 Jun;22(2):170-80.
http://www.ncbi.nlm.nih.gov/pubmed/22577003?tool=bestpractice.com
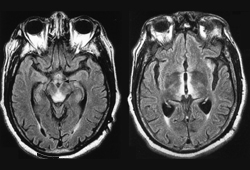
With MRI, on T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences, such abnormalities appear as signal hyperintensities in affected regions.[76]Kitaguchi T, Ota Y, Liao E, et al. The role of MRI in the prognosis of Wernicke's encephalopathy. J Neuroimaging. 2023 Nov-Dec;33(6):917-25.
https://onlinelibrary.wiley.com/doi/10.1111/jon.13143
http://www.ncbi.nlm.nih.gov/pubmed/37355834?tool=bestpractice.com
Typical MRI findings in patients with Wernicke encephalopathy include symmetrical signal abnormalities in the periventricular thalamic region, hypothalamus, mammillary bodies, periaqueductal region, and floor of the fourth ventricle.[76]Kitaguchi T, Ota Y, Liao E, et al. The role of MRI in the prognosis of Wernicke's encephalopathy. J Neuroimaging. 2023 Nov-Dec;33(6):917-25.
https://onlinelibrary.wiley.com/doi/10.1111/jon.13143
http://www.ncbi.nlm.nih.gov/pubmed/37355834?tool=bestpractice.com
The imaging characteristics of Wernicke encephalopathy may differ in patients without concurrent alcohol-use disorder. Selective involvement of cranial nerve nuclei, cerebellum, red nuclei, dentate nuclei, fornix, splenium, cerebral cortex, and basal ganglia typically characterize imagining in patients with nonalcoholic-related Wernicke encephalopathy. In patients with a history of alcohol-use disorder, lesions may more commonly demonstrate contrast enhancement in the mamillary bodies and thalamus.[77]Zuccoli G, Santa Cruz D, Bertolini M, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009 Jan;30(1):171-6.
https://www.ajnr.org/content/30/1/171
http://www.ncbi.nlm.nih.gov/pubmed/18945789?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Fluid attenuated inversion recovery (FLAIR) MRI brain of patient with Wernicke encephalopathyRamulu P, Moghekar A, Chaudhry V, et al, Wernicke's encephalopathy, Neurology 2002;59:846; used with permission [Citation ends].