Axial spondyloarthritis
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
adults with pain and/or stiffness
nonsteroidal anti-inflammatory drug + nonpharmacologic therapy
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first-line drug treatment for patients with ankylosing spondylitis (AS) with pain and stiffness.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Inadequate dosing is a common reason for lack of response to NSAIDs. Patients should be challenged with the largest tolerated dose of an NSAID (within its recommended maximum dose), while weighing risks against benefits, before consideration is given to switching to another NSAID.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com European guidelines recommend at least two courses of NSAIDs at the maximum tolerated dose before moving on to alternative treatments.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com
Evidence that continuous NSAID use reduces progression of structural damage to the spine compared with on-demand use is conflicting.[137]Wanders A, Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005 Jun;52(6):1756-65. http://onlinelibrary.wiley.com/doi/10.1002/art.21054/full http://www.ncbi.nlm.nih.gov/pubmed/15934081?tool=bestpractice.com [138]Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012 Oct;71(10):1616-22. http://ard.bmj.com/content/71/10/1616.long http://www.ncbi.nlm.nih.gov/pubmed/22459541?tool=bestpractice.com [139]Kroon F, Landewé R, Dougados M, et al. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 Oct;71(10):1623-9. http://www.ncbi.nlm.nih.gov/pubmed/22532639?tool=bestpractice.com [140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com [141]Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016 Aug;75(8):1438-43. https://ard.bmj.com/content/75/8/1438.long http://www.ncbi.nlm.nih.gov/pubmed/26242443?tool=bestpractice.com Continuous NSAID treatment is, however, recommended in all patients with active AS on the premise that it provides symptomatic control.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Guidelines do not recommend any specific NSAID for the treatment of symptomatic AS.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com One Cochrane review concluded that traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors are effective for treatment of axSpA.[140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com
Both traditional NSAIDs and COX-2 inhibitors have been associated with an increased risk of cardiovascular morbidity.[142]Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1473048 http://www.ncbi.nlm.nih.gov/pubmed/16740558?tool=bestpractice.com COX-2 inhibitors confer a reduced risk of gastrointestinal toxicity compared with traditional NSAIDs, and coprescription of proton-pump inhibitors can reduce the risk even further. Preparations combining an NSAID with a proton-pump inhibitor are available and have demonstrated equal clinical efficacy to standard preparations.[143]Datto C, Hellmund R, Siddiqui MK. Efficacy and tolerability of naproxen/esomeprazole magnesium tablets compared with non-specific NSAIDs and COX-2 inhibitors: a systematic review and network analyses. Open Access Rheumatol Res Rev. 2013 Feb 26;5:1-19. http://www.dovepress.com/efficacy-and-tolerability-of-naproxenesomeprazole-magnesium-tablets-co-peer-reviewed-article-OARRR http://www.ncbi.nlm.nih.gov/pubmed/27790020?tool=bestpractice.com [144]Wigand R, Baerwald C, Krause A, et al. 12 years of celecoxib: an inventory. Aktuelle Rheumatologie. 2013;38:38-44. The development of acute and chronic renal failure appears to be rare. Younger patients are at lower risk for these complications. The choice of NSAID/COX-2 inhibitor should be adapted to the patient profile, and patients on regular therapy should be monitored regularly.[145]Song IH, Poddubnyy DA, Rudwaleit M, et al. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008 Apr;58(4):929-38. http://onlinelibrary.wiley.com/doi/10.1002/art.23275/full http://www.ncbi.nlm.nih.gov/pubmed/18383378?tool=bestpractice.com
Guidelines recommend supervised active physical therapy interventions over passive physical interventions (e.g., massage, heat) for patients with active AS.[115]Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016 Feb;68(2):282-98. https://onlinelibrary.wiley.com/doi/full/10.1002/art.39298 http://www.ncbi.nlm.nih.gov/pubmed/26401991?tool=bestpractice.com [116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 Stretching, strengthening, cardiopulmonary, spinal extension, and range of motion exercises are important components of the exercise program.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com Hydrotherapy may improve function and help with pain management.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [118]Liang Z, Fu C, Zhang Q, et al. Effects of water therapy on disease activity, functional capacity, spinal mobility and severity of pain in patients with ankylosing spondylitis: a systematic review and meta-analysis. Disabil Rehabil. 2021 Apr;43(7):895-902. http://www.ncbi.nlm.nih.gov/pubmed/31355676?tool=bestpractice.com Exercise type, frequency, and intensity should be tailored to the individual.[117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
Patients with AS should be routinely assessed for cardiovascular risk, and modifiable risk factors should be aggressively treated.[126]Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017 Jan;76(1):17-28. https://ard.bmj.com/content/76/1/17.long http://www.ncbi.nlm.nih.gov/pubmed/27697765?tool=bestpractice.com
Primary options
naproxen: adults: 500 mg orally twice daily, maximum 1250 mg/day
OR
naproxen/esomeprazole: adults: 375/20 mg or 500/20 mg (1 tablet) orally twice daily
OR
indomethacin: adults: 25 mg orally twice daily, maximum, 200 mg/day
OR
ibuprofen: adults: 400-800 mg orally three times daily, maximum 2400 mg/day
OR
diclofenac potassium: adults: 50 mg orally (immediate-release) three times daily
OR
celecoxib: adults: 100 mg orally twice daily, maximum 400 mg/day
analgesic
Treatment recommended for SOME patients in selected patient group
Acetaminophen or codeine may be considered in all patients who find that NSAIDs do not completely control their pain, or if NSAIDs are contraindicated and/or poorly tolerated.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com
Codeine may be used in addition to NSAIDs and/or acetaminophen if NSAIDs and/or acetaminophen is not controlling pain alone.
Primary options
acetaminophen: adults: 325-1000 mg orally every 4-6 hours, maximum 4000 mg/day
Secondary options
codeine sulfate: adults: 15-60 mg orally every 4-6 hours, maximum 360 mg/day
intra-articular corticosteroid injection
Treatment recommended for ALL patients in selected patient group
Intra-articular or local-site corticosteroid injections are recommended for localized inflammation (e.g., unilateral sacroiliitis after exclusion of infection, Achilles enthesopathy).[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com In some countries, intra-articular or local corticosteroid injection should only be considered when at least two courses of NSAIDs have failed to control symptoms.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com
Local-site corticosteroids are given in addition to NSAIDs and analgesia (and if necessary disease-modifying antirheumatic drugs [DMARDs]) for concomitant peripheral disease.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Systemic corticosteroids are not recommended.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
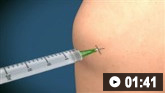
How to aspirate synovial fluid from the knee and administer intra-articular medication using a medial approach.
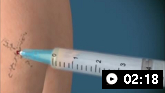
How to aspirate synovial fluid from the shoulder and administer intra-articular medication. Video demonstrates a posterior approach to the glenohumeral joint and a lateral approach to the subacromial space.
Primary options
hydrocortisone: adults: consult specialist for guidance on dose
sulfasalazine or methotrexate
Treatment recommended for ALL patients in selected patient group
Sulfasalazine and methotrexate (conventional synthetic DMARDs) may be considered for patients with peripheral disease, but there is no evidence supporting their efficacy for treating axial disease.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com They are given in addition to analgesia for concomitant peripheral disease.
Evidence for sulfasalazine efficacy in patients with peripheral disease is primarily derived from placebo-controlled randomized controlled trials conducted in the 1990s or earlier.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com One systematic review and meta-analysis noted that none of these trials assessed contemporary outcome measures (i.e., Bath Ankylosing Spondylitis Disease Activity Index [BASDAI], Bath Ankylosing Spondylitis Function Index [BASFI], Bath Ankylosing Spondylitis Metrology Index [BASMI], radiographic progression) and concluded that there is 'insufficient evidence to support any benefit of sulfasalazine in reducing pain, disease activity, radiographic progression, or improving physical function and spinal mobility in the treatment of AS'.[147]Chen J, Lin S, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev. 2014 Nov 27;(11):CD004800. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004800.pub3/full http://www.ncbi.nlm.nih.gov/pubmed/25427435?tool=bestpractice.com
There is no confirmed benefit with methotrexate in the treatment of AS.[148]Chen J, Veras MM, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev. 2013 Feb 28;(2):CD004524. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004524.pub4/full http://www.ncbi.nlm.nih.gov/pubmed/23450553?tool=bestpractice.com [149]Yang Z, Zhao W, Liu W, et al. Efficacy evaluation of methotrexate in the treatment of ankylosing spondylitis using meta-analysis. Int J Clin Pharmacol Ther. 2014 May;52(5):346-51. http://www.ncbi.nlm.nih.gov/pubmed/24618070?tool=bestpractice.com However, guidance now advocates its use in peripheral arthritis, as some studies may have used suboptimal doses of methotrexate.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Blood test monitoring is required.
Primary options
sulfasalazine: adults: 500 mg orally once daily for 1 week, then 500 mg twice daily for 1 week, then 1000 mg in the morning and 500 mg at night for 1 week, then 1000 mg twice daily
OR
methotrexate: adults: 7.5 mg orally once weekly on the same day of each week initially, increase gradually according to response, maximum 25 mg/week
adults without pain and/or stiffness
re-assessment and observation
Patients with a diagnosis of AS but without spinal pain and/or stiffness should be reviewed to confirm a definite diagnosis of AS.
Patients should be advised to keep active and to continue physical therapy exercises. On-demand treatment with NSAIDs is recommended over continuous treatment with NSAIDs for stable AS.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
children
nonsteroidal anti-inflammatory drug + nonpharmacologic measures
Oligoarthritis can be managed with a combination of NSAIDs and intra-articular corticosteroid injections.
How to aspirate synovial fluid from the knee and administer intra-articular medication using a medial approach.
How to aspirate synovial fluid from the shoulder and administer intra-articular medication. Video demonstrates a posterior approach to the glenohumeral joint and a lateral approach to the subacromial space.
The lowest effective NSAID dose with the shortest treatment duration should be used.
Guidelines recommend supervised active physical therapy interventions over passive physical interventions (e.g., massage, heat) for patients with active AS.[115]Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016 Feb;68(2):282-98. https://onlinelibrary.wiley.com/doi/full/10.1002/art.39298 http://www.ncbi.nlm.nih.gov/pubmed/26401991?tool=bestpractice.com [116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 Stretching, strengthening, cardiopulmonary, spinal extension, and range of motion exercises are important components of the exercise program.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com Hydrotherapy may improve function and help with pain management.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [118]Liang Z, Fu C, Zhang Q, et al. Effects of water therapy on disease activity, functional capacity, spinal mobility and severity of pain in patients with ankylosing spondylitis: a systematic review and meta-analysis. Disabil Rehabil. 2021 Apr;43(7):895-902. http://www.ncbi.nlm.nih.gov/pubmed/31355676?tool=bestpractice.com Exercise type, frequency, and intensity should be tailored to the individual.[117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
Primary options
naproxen: children: 5 mg/kg orally twice daily, maximum 1000 mg/day
OR
ibuprofen: children: 10 mg/kg orally every 6 hours, maximum 40 mg/kg/day
intra-articular corticosteroid injection
Treatment recommended for ALL patients in selected patient group
Oligoarthritis can be managed with a combination of NSAIDs and intra-articular corticosteroid injections.
How to aspirate synovial fluid from the knee and administer intra-articular medication using a medial approach.
How to aspirate synovial fluid from the shoulder and administer intra-articular medication. Video demonstrates a posterior approach to the glenohumeral joint and a lateral approach to the subacromial space.
Primary options
hydrocortisone: children: consult specialist for guidance on dose
sulfasalazine + nonpharmacologic measures
Sulfasalazine may be used in children >6 years of age.
Persistent oligoarthritis or polyarthritis is commonly treated with sulfasalazine or methotrexate.[195]Burgos-Vargas R, Vazquez-Mellado J, Pacheco-Tena C, et al. A 26 week randomised, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Ann Rheum Dis. 2002 Oct;61(10):941-2. http://ard.bmj.com/content/61/10/941.long http://www.ncbi.nlm.nih.gov/pubmed/12228171?tool=bestpractice.com The use of methotrexate is based largely on efficacy data from other subtypes of juvenile idiopathic arthritis.[196]Kemper AR, Van Mater HA, Coeytaux RR, et al. Systematic review of disease-modifying antirheumatic drugs for juvenile idiopathic arthritis. BMC Pediatr. 2012 Mar 15;12:29. http://bmcpediatr.biomedcentral.com/articles/10.1186/1471-2431-12-29 http://www.ncbi.nlm.nih.gov/pubmed/22420649?tool=bestpractice.com [197]Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA. 2005 Oct 5;294(13):1671-84. http://www.ncbi.nlm.nih.gov/pubmed/16204667?tool=bestpractice.com
Guidelines recommend supervised active physical therapy interventions over passive physical interventions (e.g., massage, heat) for patients with active AS.[115]Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016 Feb;68(2):282-98. https://onlinelibrary.wiley.com/doi/full/10.1002/art.39298 http://www.ncbi.nlm.nih.gov/pubmed/26401991?tool=bestpractice.com [116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 Stretching, strengthening, cardiopulmonary, spinal extension, and range of motion exercises are important components of the exercise program.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com Hydrotherapy may improve function and help with pain management.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [118]Liang Z, Fu C, Zhang Q, et al. Effects of water therapy on disease activity, functional capacity, spinal mobility and severity of pain in patients with ankylosing spondylitis: a systematic review and meta-analysis. Disabil Rehabil. 2021 Apr;43(7):895-902. http://www.ncbi.nlm.nih.gov/pubmed/31355676?tool=bestpractice.com Exercise type, frequency, and intensity should be tailored to the individual.[117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
Primary options
sulfasalazine: children >6 years of age: 10 mg/kg/day orally given in 2 divided doses initially, increase gradually according to response, maximum 50 mg/kg/day given in 2-4 divided doses
tumor necrosis factor (TNF)-alpha inhibitor + nonpharmacologic measures
Enthesitis (inflammation of the tendon or ligament attachments to bone) may respond to local corticosteroid injections under radiographic guidance.
Adalimumab improved signs and symptoms of juvenile enthesitis-related arthritis at 12 weeks, sustained through 52 weeks, in one small placebo-controlled randomized trial.[198]Burgos-Vargas R, Tse SM, Horneff G, et al. A randomized, double-blind, placebo-controlled multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res (Hoboken). 2015 Nov;67(11):1503-12. https://onlinelibrary.wiley.com/doi/full/10.1002/acr.22657 http://www.ncbi.nlm.nih.gov/pubmed/26223543?tool=bestpractice.com The safety profile was consistent with previous adalimumab studies.[198]Burgos-Vargas R, Tse SM, Horneff G, et al. A randomized, double-blind, placebo-controlled multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res (Hoboken). 2015 Nov;67(11):1503-12. https://onlinelibrary.wiley.com/doi/full/10.1002/acr.22657 http://www.ncbi.nlm.nih.gov/pubmed/26223543?tool=bestpractice.com
Etanercept showed sustained efficacy at treating clinical symptoms over 96 weeks, with no major safety issues, in one multicenter open-label study of children with subtypes of juvenile arthritis (including spondyloarthritis).[199]Constantin T, Foeldvari I, Vojinovic J, et al. Paediatric Rheumatology International Trials Organisation (PRINTO). Two-year efficacy and safety of etanercept in pediatric patients with extended oligoarthritis, enthesitis-related arthritis, or psoriatic arthritis. J Rheumatol. 2016 Apr;43(4):816-24. http://www.ncbi.nlm.nih.gov/pubmed/26932344?tool=bestpractice.com
Specific risks have been identified for patients treated with biologic DMARDs, including TNF-alpha inhibitors, therefore there are precautions that clinicians should take before initiating treatment. A guideline from the British Society of Rheumatology (BSR) outlines recommendations on precautions for biologic DMARD use.[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com The guideline recommends that baseline screening for patients with AS prior to treatment should include complete blood count; creatinine/calculated glomerular filtration rate; alanine aminotransferase and/or aspartate aminotransferase; albumin; tuberculin skin test or interferon-gamma release assay or both as appropriate; hepatitis B and C serology; chest radiograph.[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com The BSR recommends that treatment with biologic DMARDs should not be initiated in the presence of serious active infections (defined as requiring intravenous antibiotics or hospitalization; not including tuberculosis).[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com For patients at a high risk of infection, biologic DMARDs should be used with caution after discussing risks and benefits. Etanercept should be considered as a first-line biologic therapy in patients at high risk of infection.[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com
Guidelines recommend supervised active physical therapy interventions over passive physical interventions (e.g., massage, heat) for patients with active AS.[115]Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016 Feb;68(2):282-98. https://onlinelibrary.wiley.com/doi/full/10.1002/art.39298 http://www.ncbi.nlm.nih.gov/pubmed/26401991?tool=bestpractice.com [116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 Stretching, strengthening, cardiopulmonary, spinal extension, and range of motion exercises are important components of the exercise program.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com Hydrotherapy may improve function and help with pain management.[116]National Institute for Health and Care Excellence (UK). Spondyloarthritis in over 16s: diagnosis and management. Jun 2017 [internet publication]. https://www.nice.org.uk/guidance/ng65 [118]Liang Z, Fu C, Zhang Q, et al. Effects of water therapy on disease activity, functional capacity, spinal mobility and severity of pain in patients with ankylosing spondylitis: a systematic review and meta-analysis. Disabil Rehabil. 2021 Apr;43(7):895-902. http://www.ncbi.nlm.nih.gov/pubmed/31355676?tool=bestpractice.com Exercise type, frequency, and intensity should be tailored to the individual.[117]Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum. 2016 Feb;45(4):411-27. https://www.sciencedirect.com/science/article/pii/S0049017215002012 http://www.ncbi.nlm.nih.gov/pubmed/26493464?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
Primary options
etanercept: children: consult specialist for guidance on dose
OR
infliximab: children: consult specialist for guidance on dose
OR
adalimumab: children: consult specialist for guidance on dose
adults with pain and/or stiffness refractory to 2 NSAIDs and nonpharmacologic measures
tumor necrosis factor (TNF)-alpha inhibitor + physical therapy
Biologic DMARDs should be considered in patients with axial disease activity despite conventional treatment (including NSAIDs and nonpharmacologic treatment).[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com For patients with peripheral disease biologic DMARDs may be considered when conventional treatment including local corticosteroid injection or sulfasalazine is ineffective or contraindicated.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com
Specific risks have been identified for patients treated with biologic DMARDs, including TNF-alpha inhibitors, therefore there are precautions that clinicians should take before initiating treatment. A guideline from the BSR outlines recommendations on precautions for biologic DMARD use.[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com The guideline recommends that baseline screening for patients with AS prior to treatment should include complete blood count; creatinine/calculated glomerular filtration rate; alanine aminotransferase and/or aspartate aminotransferase; albumin; tuberculin skin test or interferon-gamma release assay or both as appropriate; hepatitis B and C serology; chest radiograph.[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com The BSR recommends that treatment with biologic DMARDs should not be initiated in the presence of serious active infections (defined as requiring intravenous antibiotics or hospitalization; not including tuberculosis).[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com For patients at a high risk of infection, biologic DMARDs should be used with caution after discussing risks and benefits. Etanercept should be considered as a first-line biologic therapy in patients at high risk of infection.
Adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab (all TNF-alpha inhibitors) are recommended as first-line treatment after conventional treatment has failed.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com Guidelines recommend monoclonal antibodies over etanercept for the treatment of patients with AS and recurrent uveitis, or with AS and inflammatory bowel disease.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Adalimumab is a recombinant monoclonal antibody that binds specifically to TNF and neutralizes the biologic function of TNF. Subsequent to completing a 24-week randomized controlled trial, approximately half of patients with active AS who received open-label adalimumab experienced sustained remission during a 5-year follow-up period.[162]Sieper J, van der HD, Dougados M, et al. Early response to adalimumab predicts long-term remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 May;71(5):700-6. http://ard.bmj.com/content/71/5/700.full http://www.ncbi.nlm.nih.gov/pubmed/22128084?tool=bestpractice.com The strongest predictor of remission was achievement of remission at 12 weeks of treatment.[162]Sieper J, van der HD, Dougados M, et al. Early response to adalimumab predicts long-term remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 May;71(5):700-6. http://ard.bmj.com/content/71/5/700.full http://www.ncbi.nlm.nih.gov/pubmed/22128084?tool=bestpractice.com Data from nearly 12 years of exposure in clinical trials suggest that risk of serious opportunistic infection and malignancy is reduced in patients prescribed adalimumab for AS compared with those prescribed adalimumab for rheumatoid arthritis.[163]Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis. 2013 Apr;72(4):517-24. https://ard.bmj.com/content/72/4/517.long http://www.ncbi.nlm.nih.gov/pubmed/22562972?tool=bestpractice.com
Certolizumab pegol is a pegylated humanized monoclonal antibody directed against TNF-alpha. In one 52-week study, patients with nonradiographic axial spondyloarthropathy (nr-axSpA) receiving certolizumab pegol were almost seven times more likely to achieve major improvement in the AS Disease Activity Score (ASDAS) compared with placebo.[164]Deodhar A, Gensler LS, Kay J, et al. A fifty-two-week, randomized, placebo-controlled trial of certolizumab pegol in nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Jul;71(7):1101-11. https://onlinelibrary.wiley.com/doi/full/10.1002/art.40866 http://www.ncbi.nlm.nih.gov/pubmed/30848558?tool=bestpractice.com
Sustained response to certolizumab pegol over a 4-year period has been reported in a long-term study of patients with axSpA.[165]van der Heijde D, Dougados M, Landewé R, et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology (Oxford). 2017 Sep 1;56(9):1498-509. https://academic.oup.com/rheumatology/article/56/9/1498/3819409 http://www.ncbi.nlm.nih.gov/pubmed/28498975?tool=bestpractice.com Treatment with certolizumab reduced radiographic progression and spinal inflammation.[166]van der Heijde D, Baraliakos X, Hermann KA, et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis. 2018 May;77(5):699-705. http://www.ncbi.nlm.nih.gov/pubmed/29343510?tool=bestpractice.com There is some evidence that patients with early axSpA with sustained remission (at 48 weeks) can reduce their dose of certolizumab pegol; treatment should not be discontinued due to the high risk of flare following certolizumab pegol withdrawal.[167]Landewé RB, van der Heijde D, Dougados M, et al. Maintenance of clinical remission in early axial spondyloarthritis following certolizumab pegol dose reduction. Ann Rheum Dis. 2020 Jul;79(7):920-8. https://ard.bmj.com/content/79/7/920.long http://www.ncbi.nlm.nih.gov/pubmed/32381562?tool=bestpractice.com
Etanercept is a human TNF receptor p75 Fc fusion protein. It is a competitive inhibitor of TNF binding to its cell surface receptors.
Long-term etanercept may improve clinical and imaging outcomes in patients with early active axSpA.[168]Dougados M, van der Heijde D, Sieper J, et al. Effects of long-term etanercept treatment on clinical outcomes and objective signs of inflammation in early nonradiographic axial spondyloarthritis: 104-week results from a randomized, placebo-controlled study. Arthritis Care Res (Hoboken). 2017 Oct;69(10):1590-8. https://onlinelibrary.wiley.com/doi/full/10.1002/acr.23276 http://www.ncbi.nlm.nih.gov/pubmed/28482137?tool=bestpractice.com [169]Rios Rodriguez V, Hermann KG, Weiß A, et al. Progression of structural damage in the sacroiliac joints in patients with early axial spondyloarthritis during long-term anti-tumor necrosis factor treatment: six-year results of continuous treatment with etanercept. Arthritis Rheumatol. 2019 May;71(5):722-8. http://www.ncbi.nlm.nih.gov/pubmed/30625261?tool=bestpractice.com However, results from one small randomized controlled trial indicate that in patients with suspected nr-axSpA with high disease activity, etanercept is no more effective than placebo at 16 weeks.[170]Rusman T, van der Weijden MAC, Nurmohamed MT, et al. Is treatment in patients with suspected nonradiographic axial spondyloarthritis effective? Six-month results of a placebo-controlled trial. Arthritis Rheumatol. 2021 May;73(5):806-15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8251708 http://www.ncbi.nlm.nih.gov/pubmed/33277982?tool=bestpractice.com
Golimumab is a human monoclonal antibody that prevents the binding of TNF-alpha to its receptors.
Long-term studies report sustained efficacy of golimumab in the treatment of active AS through 24, 52, and 104 weeks.[173]Braun J, Deodhar A, Inman RD, et al. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis. 2012 May;71(5):661-7. http://ard.bmj.com/content/71/5/661.full http://www.ncbi.nlm.nih.gov/pubmed/22012970?tool=bestpractice.com [174]van der Heijde D, Deodhar A, Braun J, et al. The effect of golimumab therapy on disease activity and health-related quality of life in patients with ankylosing spondylitis: 2-year results of the GO-RAISE trial. J Rheumatol. 2014 Jun;41(6):1095-103. http://www.ncbi.nlm.nih.gov/pubmed/24737912?tool=bestpractice.com [175]Reveille JD, Deodhar A, Caldron PH, et al. Safety and efficacy of intravenous golimumab in adults with ankylosing spondylitis: results through 1 year of the GO-ALIVE study. J Rheumatol. 2019 Oct;46(10):1277-83. https://www.jrheum.org/content/46/10/1277.long http://www.ncbi.nlm.nih.gov/pubmed/30824635?tool=bestpractice.com
Pooled 5-year safety data from clinical trials of rheumatoid arthritis, psoriatic arthritis, and AS suggest a numerically increased incidence of tuberculosis, opportunistic infection, lymphoma, and demyelination, with a higher dose of golimumab compared with a lower dose of golimumab.[176]Kay J, Fleischmann R, Keystone E, et al. Five-year safety data from 5 clinical trials of subcutaneous golimumab in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2016 Dec;43(12):2120-30. http://www.ncbi.nlm.nih.gov/pubmed/27803138?tool=bestpractice.com The majority of treated patients (67%) participated in rheumatoid arthritis trials.
Infliximab is a chimeric immunoglobulin G1 monoclonal antibody that binds with high affinity to TNF-alpha.
In patients with AS, treatment response with infliximab is sustained over the long term.[177]Heldmann F, Baraliakos X, Kiltz U, et al. Clinical experience with the European Ankylosing Spondylitis Infliximab Cohort (EASIC): long-term extension over 7 years with focus on clinical efficacy and safety. Clin Exp Rheumatol. 2016 Mar-Apr;34(2):184-90. http://www.ncbi.nlm.nih.gov/pubmed/27049733?tool=bestpractice.com [178]Elalouf O, Elkayam O. Long-term safety and efficacy of infliximab for the treatment of ankylosing spondylitis. Ther Clin Risk Manag. 2015 Nov 19;11:1719-26. https://www.dovepress.com/long-term-safety-and-efficacy-of-infliximab-for-the-treatment-of-ankyl-peer-reviewed-fulltext-article-TCRM http://www.ncbi.nlm.nih.gov/pubmed/26640380?tool=bestpractice.com [179]Kobayashi S, Yoshinari T. A multicenter, open-label, long-term study of three-year infliximab administration in Japanese patients with ankylosing spondylitis. Mod Rheumatol. 2017 Jan;27(1):142-9. http://www.ncbi.nlm.nih.gov/pubmed/27299733?tool=bestpractice.com In one network meta-analysis, infliximab was demonstrated to be the most effective TNF-alpha inhibitor, with the highest probability of patients achieving ASAS20 response both at 12 and 24 weeks of treatment.[180]Migliore A, Gigliucci G, Integlia D, et al. Differences in biologics for treating ankylosing spondylitis: the contribution of network meta-analysis. Eur Rev Med Pharmacol Sci. 2021 Jan;25(1):56-64. https://www.europeanreview.org/article/24347 http://www.ncbi.nlm.nih.gov/pubmed/33506892?tool=bestpractice.com
Proactive therapeutic drug monitoring of infliximab (proposed as an alternative to standard therapy to maximize efficacy and safety of biologic agents) did not significantly improve clinical remission rates over 30 weeks in patients with chronic immune-mediated inflammatory diseases including spondyloarthritis.[181]Syversen SW, Goll GL, Jørgensen KK, et al. Effect of therapeutic drug monitoring vs standard therapy during infliximab induction on disease remission in patients with chronic immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021 May 4;325(17):1744-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8097498 http://www.ncbi.nlm.nih.gov/pubmed/33944876?tool=bestpractice.com
US guidelines recommend against tapering of biologic agent dose as a standard approach in patients with stable AS disease.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com European guidance suggests that tapering of a biologic DMARD can be considered in patients in sustained remission.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com One systematic review reported that patient-tailored dose reduction of TNF-alpha inhibitors successfully preserved a stable low disease activity in with remission rates ranging between 20.2% and 93.7%.[156]Saoussen M, Yasmine M, Lilia N, et al. Tapering biologics in axial spondyloarthritis: a systematic literature review. Int Immunopharmacol. 2022 Nov;112:109256. http://www.ncbi.nlm.nih.gov/pubmed/36150228?tool=bestpractice.com However a complete treatment discontinuation is associated with a high risk of flares.[156]Saoussen M, Yasmine M, Lilia N, et al. Tapering biologics in axial spondyloarthritis: a systematic literature review. Int Immunopharmacol. 2022 Nov;112:109256. http://www.ncbi.nlm.nih.gov/pubmed/36150228?tool=bestpractice.com [157]Uhrenholt L, Christensen R, Dinesen WKH, et al. Risk of flare after tapering or withdrawal of biologic/targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis or axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2022 Aug 3;61(8):3107-22. https://academic.oup.com/rheumatology/article/61/8/3107/6448788 http://www.ncbi.nlm.nih.gov/pubmed/34864896?tool=bestpractice.com
Reported adverse effects of TNF-alpha inhibitors (mainly derived from rheumatoid arthritis studies, where many of the increased risks are at least partly attributable to the underlying rheumatologic disorder) include serious infections, the development of malignancies such as lymphoma, worsening of cardiac failure, and a low incidence of demyelinating disease.[206]Dixon WG, Symmons DP, Lunt M, et al; British Society for Rheumatology Biologics Register Control Centre Consortium, Silman AJ; British Society for Rheumatology Biologics Register. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007 Sep;56(9):2896-904. http://onlinelibrary.wiley.com/doi/10.1002/art.22808/full http://www.ncbi.nlm.nih.gov/pubmed/17763441?tool=bestpractice.com [207]Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003 Nov;48(11):3013-22. http://onlinelibrary.wiley.com/doi/10.1002/art.11301/full http://www.ncbi.nlm.nih.gov/pubmed/14613261?tool=bestpractice.com [208]Minozzi S, Bonovas S, Lytras T, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016 Dec;15(suppl 1):11-34. https://air.unimi.it/retrieve/handle/2434/481066/797018/Anti_TNF_Infections.pdf http://www.ncbi.nlm.nih.gov/pubmed/27924643?tool=bestpractice.com [209]Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002 Dec;46(12):3151-8. http://onlinelibrary.wiley.com/doi/10.1002/art.10679/full http://www.ncbi.nlm.nih.gov/pubmed/12483718?tool=bestpractice.com [210]Nannini C, Cantini F, Niccoli L, et al. Single-center series and systematic review of randomized controlled trials of malignancies in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis receiving anti-tumor necrosis factor alpha therapy: is there a need for more comprehensive screening procedures? Arthritis Rheum. 2009 Jun 15;61(6):801-12. https://onlinelibrary.wiley.com/doi/full/10.1002/art.24506 http://www.ncbi.nlm.nih.gov/pubmed/19479708?tool=bestpractice.com [211]Chung ES, Packer M, Lo KH, et al; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003 Jul 1;107(25):3133-40. http://circ.ahajournals.org/content/107/25/3133.full http://www.ncbi.nlm.nih.gov/pubmed/12796126?tool=bestpractice.com [212]Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001 Dec;44(12):2862-9. http://www.ncbi.nlm.nih.gov/pubmed/11762947?tool=bestpractice.com The risks of adverse events may be lower in patients with AS than in patients with rheumatoid arthritis.[213]Burmester GR, Mease P, Dijkmans BA, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009 Dec;68(12):1863-9. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2770105 http://www.ncbi.nlm.nih.gov/pubmed/19147611?tool=bestpractice.com Systematic reviews report no significant increase in the risk of infection in patients with AS on TNF-alpha inhibitors.[214]Fouque-Aubert A, Jette-Paulin L, Combescure C, et al. Serious infections in patients with ankylosing spondylitis with and without TNF blockers: a systematic review and meta-analysis of randomised placebo-controlled trials. Ann Rheum Dis. 2010 Oct;69(10):1756-61. http://www.ncbi.nlm.nih.gov/pubmed/19640854?tool=bestpractice.com [215]Hou LQ, Jiang GX, Chen YF, et al. The comparative safety of TNF inhibitors in ankylosing spondylitis-a meta-analysis update of 14 randomized controlled trials. Clin Rev Allergy Immunol. 2018 Apr;54(2):234-43. http://www.ncbi.nlm.nih.gov/pubmed/28717941?tool=bestpractice.com [216]Ma Z, Liu X, Xu X, et al. Safety of tumor necrosis factor-alpha inhibitors for treatment of ankylosing spondylitis: a meta-analysis. Medicine (Baltimore). 2017 Jun;96(25):e7145. https://journals.lww.com/md-journal/Fulltext/2017/06230/Safety_of_tumor_necrosis_factor_alpha_inhibitors.17.aspx http://www.ncbi.nlm.nih.gov/pubmed/28640088?tool=bestpractice.com
TNF-alpha inhibitors are contraindicated in moderate-to-severe heart failure and should be avoided in New York Heart Association class IV cardiac failure, active tuberculosis and other serious infections, and in patients with a history of demyelinating disease or malignancy (particularly melanoma). Before initiation of therapy, evidence of prior hepatitis B virus infection should be sought. Data suggest that patients with hepatitis B surface antigen (HBsAg)-negative and anti-hepatitis B core (HBc)-positive status should be carefully monitored while undergoing treatment with TNF-alpha inhibitors to monitor for potential reactivation of the virus.[217]National Institute for Health and Care Excellence (UK). TNF-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis. Feb 2016 [internet publication]. https://www.nice.org.uk/guidance/ta383 [218]Lee YH, Bae SC, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol. 2013 Jan-Feb;31(1):118-21. http://www.clinexprheumatol.org/article.asp?a=5761 http://www.ncbi.nlm.nih.gov/pubmed/23111095?tool=bestpractice.com Evidence of active and inactive (latent) tuberculosis infection should also be sought.[217]National Institute for Health and Care Excellence (UK). TNF-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis. Feb 2016 [internet publication]. https://www.nice.org.uk/guidance/ta383 Pretreatment tuberculosis screening is particularly important in endemic populations.[219]Kumar A. Experience with anti-tumor necrosis factor-alpha therapy in India. APLAR J Rheumatol. 2006 July;9(2):136-41. http://onlinelibrary.wiley.com/doi/10.1111/j.1479-8077.2006.00188.x/full
Baseline chest x-ray and tuberculosis screening are required.[220]Provenzano G, Ferrante MC, Simon G. TB screening and anti-TNF alpha treatment. Thorax. 2005 Jul;60(7):613. http://thorax.bmj.com/content/60/7/613.1.long http://www.ncbi.nlm.nih.gov/pubmed/15994274?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
Primary options
adalimumab: adults: 40 mg subcutaneously every 2 weeks
OR
etanercept: adults: 50 mg subcutaneously once weekly
OR
golimumab: adults: 50 mg subcutaneously once monthly; 2 mg/kg intravenously at 0 and 4 weeks, followed by every 8 weeks thereafter
OR
infliximab: adults: 5 mg/kg intravenously at 0, 2, and 6 weeks, followed by every 6 weeks thereafter
OR
certolizumab pegol: adults: 400 mg subcutaneously at weeks 0, 2, and 4, then 200 mg every 2 weeks or 400 mg every 4 weeks
continued nonsteroidal anti-inflammatory drug
Treatment recommended for SOME patients in selected patient group
On initiation of TNF-alpha inhibitor, NSAIDs are recommended for active AS disease and can be taken continuously until the patient is stable. After this, patients may use NSAIDs on demand.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Continuing NSAIDs is particularly important in patients with known risk factors for radiographic progression (i.e., presence of radiographic syndesmophytes, elevated inflammatory markers, smoking history).
Evidence that continuous NSAID use reduces progression of structural damage to the spine compared with on-demand use is conflicting.[137]Wanders A, Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005 Jun;52(6):1756-65. http://onlinelibrary.wiley.com/doi/10.1002/art.21054/full http://www.ncbi.nlm.nih.gov/pubmed/15934081?tool=bestpractice.com [138]Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012 Oct;71(10):1616-22. http://ard.bmj.com/content/71/10/1616.long http://www.ncbi.nlm.nih.gov/pubmed/22459541?tool=bestpractice.com [139]Kroon F, Landewé R, Dougados M, et al. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 Oct;71(10):1623-9. http://www.ncbi.nlm.nih.gov/pubmed/22532639?tool=bestpractice.com [140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com [141]Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016 Aug;75(8):1438-43. https://ard.bmj.com/content/75/8/1438.long http://www.ncbi.nlm.nih.gov/pubmed/26242443?tool=bestpractice.com Continuous NSAID treatment is, however, recommended in all patients with active AS on the premise that it provides symptomatic control.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Guidelines do not recommend any specific NSAID for the treatment of symptomatic AS.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com One Cochrane review concluded that traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors are effective for treatment of axSpA.[140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com
Both traditional NSAIDs and COX-2 inhibitors have been associated with an increased risk of cardiovascular morbidity.[142]Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1473048 http://www.ncbi.nlm.nih.gov/pubmed/16740558?tool=bestpractice.com COX-2 inhibitors confer a reduced risk of gastrointestinal toxicity compared with traditional NSAIDs, and coprescription of proton-pump inhibitors can reduce the risk even further. Preparations combining an NSAID with a proton-pump inhibitor are available and have demonstrated equal clinical efficacy to standard preparations.[143]Datto C, Hellmund R, Siddiqui MK. Efficacy and tolerability of naproxen/esomeprazole magnesium tablets compared with non-specific NSAIDs and COX-2 inhibitors: a systematic review and network analyses. Open Access Rheumatol Res Rev. 2013 Feb 26;5:1-19. http://www.dovepress.com/efficacy-and-tolerability-of-naproxenesomeprazole-magnesium-tablets-co-peer-reviewed-article-OARRR http://www.ncbi.nlm.nih.gov/pubmed/27790020?tool=bestpractice.com [144]Wigand R, Baerwald C, Krause A, et al. 12 years of celecoxib: an inventory. Aktuelle Rheumatologie. 2013;38:38-44. The development of acute and chronic renal failure appears to be rare. Younger patients are at lower risk for these complications. The choice of NSAID/COX-2 inhibitor should be adapted to the patient profile, and patients on regular therapy should be monitored regularly.[145]Song IH, Poddubnyy DA, Rudwaleit M, et al. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008 Apr;58(4):929-38. http://onlinelibrary.wiley.com/doi/10.1002/art.23275/full http://www.ncbi.nlm.nih.gov/pubmed/18383378?tool=bestpractice.com
Primary options
naproxen: adults: 500 mg orally twice daily, maximum 1250 mg/day
OR
naproxen/esomeprazole: adults: 375/20 mg or 500/20 mg (1 tablet) orally twice daily
OR
indomethacin: adults: 25 mg orally twice daily, maximum, 200 mg/day
OR
ibuprofen: adults: 400-800 mg orally three times daily, maximum 2400 mg/day
OR
diclofenac potassium: adults: 50 mg orally (immediate-release) three times daily, maximum 150 mg/day
OR
celecoxib: adults: 100 mg orally twice daily, maximum 400 mg/day
interleukin-17 inhibitor + physical therapy
If TNF-alpha inhibitor therapy fails, switching to an interleukin (IL)-17 inhibitor (e.g., secukinumab, ixekizumab) should be considered.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com For patients with significant psoriasis, an IL-17 inhibitor are preferred treatment over TNF-alpha-inhibitors.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com
IL-17 inhibitors have proven efficacy in patients who experience treatment failure with a TNF-alpha inhibitor, but improvements may be greater in TNF-alpha inhibitor naive patients.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [182]Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 Study. Ann Rheum Dis. 2017 Mar;76(3):571-92. http://www.ncbi.nlm.nih.gov/pubmed/27582421?tool=bestpractice.com
Specific risks have been identified for patients treated with biologic DMARDs, including IL-17 inhibitors, therefore there are precautions that clinicians should take before initiating treatment. A guideline from the BSR outlines recommendations on precautions for biologic DMARD use.[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com The guideline recommends that baseline screening for patients with AS prior to treatment should include complete blood count; creatinine/calculated glomerular filtration rate; alanine aminotransferase and/or aspartate aminotransferase; albumin; tuberculin skin test or interferon-gamma release assay or both as appropriate; hepatitis B and C serology; chest radiograph.[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com The BSR recommends that treatment with biologic DMARDs should not be initiated in the presence of serious active infections (defined as requiring intravenous antibiotics or hospitalization; not including tuberculosis).[155]Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford). 2019 Feb 1;58(2):e3-42. https://academic.oup.com/rheumatology/article/58/2/e3/5076446 http://www.ncbi.nlm.nih.gov/pubmed/30137552?tool=bestpractice.com For patients at a high risk of infection, biologic DMARDs should be used with caution after discussing risks and benefits.
Guidelines recommend an IL-17 inhibitor for patients with a primary nonresponse (absence of clinically meaningful improvement in disease activity over 3 to 6 months after treatment initiation) to the first TNF-alpha inhibitor.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Secukinumab is a fully humanized anti-IL-17A monoclonal antibody. IL-17 is a cytokine produced by T-helper 17 cells that has been increasingly implicated in a variety of autoimmune and inflammatory diseases.
Secukinumab significantly reduces symptoms and signs of AS, as measured by Assessment of SpondyloArthritis International Society criteria.[184]Baeten D, Sieper J, Braun J, et al; MEASURE 1 Study Group, MEASURE 2 Study Group. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015 Dec 24;373(26):2534-48. http://www.nejm.org/doi/pdf/10.1056/NEJMoa1505066 http://www.ncbi.nlm.nih.gov/pubmed/26699169?tool=bestpractice.com [185]Deodhar A, Blanco R, Dokoupilová E, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021 Jan;73(1):110-20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7839589 http://www.ncbi.nlm.nih.gov/pubmed/32770640?tool=bestpractice.com
Sustained secukinumab efficacy (signs and symptoms, low rate of radiographic progression) over 4 to 5 years has been reported in patients with AS.[186]Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology (Oxford). 2019 May 1;58(5):859-68. https://academic.oup.com/rheumatology/article/58/5/859/5253847 http://www.ncbi.nlm.nih.gov/pubmed/30590813?tool=bestpractice.com [187]Baraliakos X, Braun J, Deodhar A, et al. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD Open. 2019 Sep 3;5(2):e001005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6744073 http://www.ncbi.nlm.nih.gov/pubmed/31565244?tool=bestpractice.com
Ixekizumab is a recombinant humanized monoclonal antibody that binds with high affinity to IL-17A.
In a phase 3 randomized trial of patients with nr-axSpA, ixekizumab significantly improved signs and symptoms (disease activity, physical function, quality of life, and inflammation) compared with placebo at weeks 16 and 52.[189]Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020 Jan 4;395(10217):53-64. http://www.ncbi.nlm.nih.gov/pubmed/31813637?tool=bestpractice.com In an extension of this trial, patients who completed the initial 52-week phase and were randomized to continued ixekizumab experienced significantly delayed time-to-flare compared with those randomized to placebo.[190]Landewé RB, Gensler LS, Poddubnyy D, et al. Continuing versus withdrawing ixekizumab treatment in patients with axial spondyloarthritis who achieved remission: efficacy and safety results from a placebo-controlled, randomised withdrawal study (COAST-Y). Ann Rheum Dis. 2021 May 6;80(8):1022-30. https://ard.bmj.com/content/80/8/1022.long http://www.ncbi.nlm.nih.gov/pubmed/33958326?tool=bestpractice.com
Ixekizumab is recommended to treat patients who have failed TNF-alpha inhibitor therapy.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Ixekizumab is approved in Europe for this indication, but it is not yet included in European guidance for the treatment of AS.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
Primary options
secukinumab: with loading dose: 150 mg subcutaneously at weeks 0, 1, 2, 3, and 4, followed by 150 mg every 4 weeks; without loading dose: 150 mg subcutaneously every 4 weeks
More secukinumabMay be administered with or without a loading dose.
OR
ixekizumab: 160 mg subcutaneously at week 0, followed by 80 mg every 4 weeks
continued nonsteroidal anti-inflammatory drug
Treatment recommended for SOME patients in selected patient group
On initiation, NSAIDs are recommended for active AS and can be taken continuously until the patient is stable. After this, patients may use NSAIDs on demand.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Consideration of continuing NSAIDs is particularly important in patients with known risk factors for radiographic progression (i.e., presence of radiographic syndesmophytes, elevated inflammatory markers, smoking history).
Evidence that continuous NSAID use reduces progression of structural damage to the spine compared with on-demand use is conflicting.[137]Wanders A, Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005 Jun;52(6):1756-65. http://onlinelibrary.wiley.com/doi/10.1002/art.21054/full http://www.ncbi.nlm.nih.gov/pubmed/15934081?tool=bestpractice.com [138]Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012 Oct;71(10):1616-22. http://ard.bmj.com/content/71/10/1616.long http://www.ncbi.nlm.nih.gov/pubmed/22459541?tool=bestpractice.com [139]Kroon F, Landewé R, Dougados M, et al. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 Oct;71(10):1623-9. http://www.ncbi.nlm.nih.gov/pubmed/22532639?tool=bestpractice.com [140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com [141]Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016 Aug;75(8):1438-43. https://ard.bmj.com/content/75/8/1438.long http://www.ncbi.nlm.nih.gov/pubmed/26242443?tool=bestpractice.com Continuous NSAID treatment is, however, recommended in all patients with active AS on the premise that it provides symptomatic control.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Guidelines do not recommend any specific NSAID for the treatment of symptomatic AS.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com One Cochrane review concluded that traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors are effective for treatment of axSpA.[140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com
Both traditional NSAIDs and COX-2 inhibitors have been associated with an increased risk of cardiovascular morbidity.[142]Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1473048 http://www.ncbi.nlm.nih.gov/pubmed/16740558?tool=bestpractice.com COX-2 inhibitors confer a reduced risk of gastrointestinal toxicity compared with traditional NSAIDs, and coprescription of proton-pump inhibitors can reduce the risk even further. Preparations combining an NSAID with a proton-pump inhibitor are available and have demonstrated equal clinical efficacy to standard preparations.[143]Datto C, Hellmund R, Siddiqui MK. Efficacy and tolerability of naproxen/esomeprazole magnesium tablets compared with non-specific NSAIDs and COX-2 inhibitors: a systematic review and network analyses. Open Access Rheumatol Res Rev. 2013 Feb 26;5:1-19. http://www.dovepress.com/efficacy-and-tolerability-of-naproxenesomeprazole-magnesium-tablets-co-peer-reviewed-article-OARRR http://www.ncbi.nlm.nih.gov/pubmed/27790020?tool=bestpractice.com [144]Wigand R, Baerwald C, Krause A, et al. 12 years of celecoxib: an inventory. Aktuelle Rheumatologie. 2013;38:38-44. The development of acute and chronic renal failure appears to be rare. Younger patients are at lower risk for these complications. The choice of NSAID/COX-2 inhibitor should be adapted to the patient profile, and patients on regular therapy should be monitored regularly.[145]Song IH, Poddubnyy DA, Rudwaleit M, et al. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008 Apr;58(4):929-38. http://onlinelibrary.wiley.com/doi/10.1002/art.23275/full http://www.ncbi.nlm.nih.gov/pubmed/18383378?tool=bestpractice.com
Primary options
naproxen: adults: 500 mg orally twice daily, maximum 1250 mg/day
OR
naproxen/esomeprazole: adults: 375/20 mg or 500/20 mg (1 tablet) orally twice daily
OR
indomethacin: adults: 25 mg orally twice daily, maximum 200 mg/day
OR
ibuprofen: adults: 400-800 mg orally three times daily, maximum 2400 mg/day
OR
diclofenac potassium: adults: 50 mg orally (immediate-release) three times daily, maximum 150 mg/day
OR
celecoxib: adults: 100 mg orally twice daily, maximum 400 mg/day
alternative tumor necrosis factor (TNF)-alpha inhibitor + physical therapy
The American College of Rheumatology recommends that adults with secondary nonresponse (recurrence of active disease after sustained clinically meaningful improvement on treatment) to TNF-alpha inhibitor consider a different TNF-alpha inhibitor treatment, before treatment with a non-TNF inhibitor biologic.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com Evidence suggests a response to a second TNF-alpha inhibitor is possible when the first agent has not worked.[158]Conti F, Ceccarelli F, Marocchi E, et al. Switching tumour necrosis factor alpha antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period. Ann Rheum Dis. 2007 Oct;66(10):1393-7. http://www.ncbi.nlm.nih.gov/pubmed/17613555?tool=bestpractice.com [159]Lie E, van der Heijde D, Uhlig T, et al. Effectiveness of switching between TNF inhibitors in ankylosing spondylitis: data from the NOR-DMARD register. Ann Rheum Dis. 2011 Jan;70(1):157-63. http://www.ncbi.nlm.nih.gov/pubmed/21062852?tool=bestpractice.com [160]Rudwaleit M, Van den Bosch F, Kron M, et al. Effectiveness and safety of adalimumab in patients with ankylosing spondylitis or psoriatic arthritis and history of anti-tumor necrosis factor therapy. Arthritis Res Ther. 2010;12(3):R117. http://arthritis-research.biomedcentral.com/articles/10.1186/ar3054 http://www.ncbi.nlm.nih.gov/pubmed/20553600?tool=bestpractice.com [161]Deodhar A, Yu D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin Arthritis Rheum. 2017 Dec;47(3):343-50. https://www.sciencedirect.com/science/article/pii/S0049017217301117?via%3Dihub http://www.ncbi.nlm.nih.gov/pubmed/28551170?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
continued nonsteroidal anti-inflammatory drug
Treatment recommended for SOME patients in selected patient group
On initiation, NSAIDs are recommended for active AS and can be taken continuously until the patient is stable. After this, patients may use NSAIDs on demand.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Consideration of continuing NSAIDs is particularly important in patients with known risk factors for radiographic progression (i.e., presence of radiographic syndesmophytes, elevated inflammatory markers, smoking history).
Evidence that continuous NSAID use reduces progression of structural damage to the spine compared with on-demand use is conflicting.[137]Wanders A, Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005 Jun;52(6):1756-65. http://onlinelibrary.wiley.com/doi/10.1002/art.21054/full http://www.ncbi.nlm.nih.gov/pubmed/15934081?tool=bestpractice.com [138]Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012 Oct;71(10):1616-22. http://ard.bmj.com/content/71/10/1616.long http://www.ncbi.nlm.nih.gov/pubmed/22459541?tool=bestpractice.com [139]Kroon F, Landewé R, Dougados M, et al. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 Oct;71(10):1623-9. http://www.ncbi.nlm.nih.gov/pubmed/22532639?tool=bestpractice.com [140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com [141]Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016 Aug;75(8):1438-43. https://ard.bmj.com/content/75/8/1438.long http://www.ncbi.nlm.nih.gov/pubmed/26242443?tool=bestpractice.com Continuous NSAID treatment is, however, recommended in all patients with active AS on the premise that it provides symptomatic control.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Guidelines do not recommend any specific NSAID for the treatment of symptomatic AS.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com One Cochrane review concluded that traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors are effective for treatment of axSpA.[140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com
Both traditional NSAIDs and COX-2 inhibitors have been associated with an increased risk of cardiovascular morbidity.[142]Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1473048 http://www.ncbi.nlm.nih.gov/pubmed/16740558?tool=bestpractice.com COX-2 inhibitors confer a reduced risk of gastrointestinal toxicity compared with traditional NSAIDs, and coprescription of proton-pump inhibitors can reduce the risk even further. Preparations combining an NSAID with a proton-pump inhibitor are available and have demonstrated equal clinical efficacy to standard preparations.[143]Datto C, Hellmund R, Siddiqui MK. Efficacy and tolerability of naproxen/esomeprazole magnesium tablets compared with non-specific NSAIDs and COX-2 inhibitors: a systematic review and network analyses. Open Access Rheumatol Res Rev. 2013 Feb 26;5:1-19. http://www.dovepress.com/efficacy-and-tolerability-of-naproxenesomeprazole-magnesium-tablets-co-peer-reviewed-article-OARRR http://www.ncbi.nlm.nih.gov/pubmed/27790020?tool=bestpractice.com [144]Wigand R, Baerwald C, Krause A, et al. 12 years of celecoxib: an inventory. Aktuelle Rheumatologie. 2013;38:38-44. The development of acute and chronic renal failure appears to be rare. Younger patients are at lower risk for these complications. The choice of NSAID/COX-2 inhibitor should be adapted to the patient profile, and patients on regular therapy should be monitored regularly.[145]Song IH, Poddubnyy DA, Rudwaleit M, et al. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008 Apr;58(4):929-38. http://onlinelibrary.wiley.com/doi/10.1002/art.23275/full http://www.ncbi.nlm.nih.gov/pubmed/18383378?tool=bestpractice.com
Primary options
naproxen: adults: 500 mg orally twice daily, maximum 1250 mg/day
OR
naproxen/esomeprazole: adults: 375/20 mg or 500/20 mg (1 tablet) orally twice daily
OR
indomethacin: adults: 25 mg orally twice daily, maximum, 200 mg/day
OR
ibuprofen: adults: 400-800 mg orally three times daily, maximum 2400 mg/day
OR
diclofenac potassium: adults: 50 mg orally (immediate-release) three times daily, maximum 150 mg/day
OR
celecoxib: adults: 100 mg orally twice daily, maximum 400 mg/day
Janus kinase inhibitor + physical therapy
Janus kinase (JAK) inhibitors (e.g., tofacitinib, upadacitinib) can be considered for patients with AS who are unresponsive or have contraindications to both TNF-alpha inhibitors and IL-17 inhibitors.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [191]National Institute for Health and Care Excellence. Upadacitinib for treating active ankylosing spondylitis. Sep 2022 [internet publication]. https://www.nice.org.uk/guidance/TA829 [192]National Institute for Health and Care Excellence. Tofacitinib for treating active ankylosing spondylitis. Oct 2023 [internet publication]. https://www.nice.org.uk/guidance/ta920
JAK inhibitors have been demonstrated to reduce disease activity, improve physical function, emotional well-being, and social participation in patients with active AS.[193]Li S, Li F, Mao N, et al. Efficacy and safety of Janus kinase inhibitors in patients with ankylosing spondylitis: a systematic review and meta-analysis. Eur J Intern Med. 2022 Aug;102:47-53. https://www.ejinme.com/article/S0953-6205(22)00143-1/fulltext http://www.ncbi.nlm.nih.gov/pubmed/35461744?tool=bestpractice.com
Safety data for the use of JAK inhibitors is limited for patients with AS as these treatments are relatively new for this population. There is evidence to suggest that JAK-inhibitor treatment is associated with an increased risk of major adverse cardiovascular events, malignancy, venous thromboembolism, opportunistic infections, and serious infections in patients with rheumatoid arthritis.[194]Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022 Jan 27;386(4):316-26. https://www.nejm.org/doi/10.1056/NEJMoa2109927 http://www.ncbi.nlm.nih.gov/pubmed/35081280?tool=bestpractice.com
Despite the heterogeneity of physical therapy and exercise programs evaluated in randomized controlled trials, systematic reviews and meta-analyses indicate that these interventions can potentially contribute to improved function, reduced pain, and reduced disease activity in patients with AS.[119]Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018 Feb;99(2):383-9.
http://www.ncbi.nlm.nih.gov/pubmed/28860095?tool=bestpractice.com
[120]Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019 Oct 2;(10):CD011321.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011321.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31578051?tool=bestpractice.com
[121]Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD002822.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002822.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/18254008?tool=bestpractice.com
[  ]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
]
How do exercise programs compare with usual care for people with ankylosing spondylitis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2829/fullShow me the answer
Primary options
tofacitinib: 5 mg orally (immediate-release) twice daily; 11 mg orally (extended-release) once daily
OR
upadacitinib: 15 mg orally once daily
continued nonsteroidal anti-inflammatory drug
Treatment recommended for SOME patients in selected patient group
On initiation, NSAIDs are recommended for active AS and can be taken continuously until the patient is stable. After this, patients may use NSAIDs on demand.[114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Consideration of continuing NSAIDs is particularly important in patients with known risk factors for radiographic progression (i.e., presence of radiographic syndesmophytes, elevated inflammatory markers, and smoking history).
Evidence that continuous NSAID use reduces progression of structural damage to the spine compared with on-demand use is conflicting.[137]Wanders A, Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005 Jun;52(6):1756-65. http://onlinelibrary.wiley.com/doi/10.1002/art.21054/full http://www.ncbi.nlm.nih.gov/pubmed/15934081?tool=bestpractice.com [138]Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012 Oct;71(10):1616-22. http://ard.bmj.com/content/71/10/1616.long http://www.ncbi.nlm.nih.gov/pubmed/22459541?tool=bestpractice.com [139]Kroon F, Landewé R, Dougados M, et al. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 Oct;71(10):1623-9. http://www.ncbi.nlm.nih.gov/pubmed/22532639?tool=bestpractice.com [140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com [141]Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016 Aug;75(8):1438-43. https://ard.bmj.com/content/75/8/1438.long http://www.ncbi.nlm.nih.gov/pubmed/26242443?tool=bestpractice.com Continuous NSAID treatment is, however, recommended in all patients with active AS on the premise that it provides symptomatic control.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com
Guidelines do not recommend any specific NSAID for the treatment of symptomatic AS.[109]Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):19-34. https://ard.bmj.com/content/82/1/19 http://www.ncbi.nlm.nih.gov/pubmed/36270658?tool=bestpractice.com [114]Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis andnonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019 Oct;71(10):1599-613. https://onlinelibrary.wiley.com/doi/full/10.1002/art.41042 http://www.ncbi.nlm.nih.gov/pubmed/31436036?tool=bestpractice.com One Cochrane review concluded that traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors are effective for treatment of axSpA.[140]Kroon FP, van der Burg LR, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015 Jul 17;(7):CD010952. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010952.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26186173?tool=bestpractice.com
Both traditional NSAIDs and COX-2 inhibitors have been associated with an increased risk of cardiovascular morbidity.[142]Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1473048 http://www.ncbi.nlm.nih.gov/pubmed/16740558?tool=bestpractice.com COX-2 inhibitors confer a reduced risk of gastrointestinal toxicity compared with traditional NSAIDs, and co-prescription of proton-pump inhibitors can reduce the risk even further. Preparations combining an NSAID with a proton-pump inhibitor are available and have demonstrated equal clinical efficacy to standard preparations.[143]Datto C, Hellmund R, Siddiqui MK. Efficacy and tolerability of naproxen/esomeprazole magnesium tablets compared with non-specific NSAIDs and COX-2 inhibitors: a systematic review and network analyses. Open Access Rheumatol Res Rev. 2013 Feb 26;5:1-19. http://www.dovepress.com/efficacy-and-tolerability-of-naproxenesomeprazole-magnesium-tablets-co-peer-reviewed-article-OARRR http://www.ncbi.nlm.nih.gov/pubmed/27790020?tool=bestpractice.com [144]Wigand R, Baerwald C, Krause A, et al. 12 years of celecoxib: an inventory. Aktuelle Rheumatologie. 2013;38:38-44. The development of acute and chronic renal failure appears to be rare. Younger patients are at lower risk for these complications. The choice of NSAID/COX-2 inhibitor should be adapted to the patient profile, and patients on regular therapy should be monitored regularly.[145]Song IH, Poddubnyy DA, Rudwaleit M, et al. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008 Apr;58(4):929-38. http://onlinelibrary.wiley.com/doi/10.1002/art.23275/full http://www.ncbi.nlm.nih.gov/pubmed/18383378?tool=bestpractice.com
Primary options
naproxen: adults: 500 mg orally twice daily, maximum 1250 mg/day
OR
naproxen/esomeprazole: adults: 375/20 mg or 500/20 mg (1 tablet) orally twice daily
OR
indomethacin: adults: 25 mg orally twice daily, maximum 200 mg/day
OR
ibuprofen: adults: 400-800 mg orally three times daily, maximum 2400 mg/day
OR
diclofenac potassium: adults: 50 mg orally (immediate-release) three times daily
OR
celecoxib: adults: 100 mg orally twice daily, maximum 400 mg/day

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer