Approach
The general approach is to address the problematic areas in the woman's sex response cycle identified during the detailed assessment.[124] Treatment is guided by the known strong correlation between women's sexual satisfaction and their mental health, including self-image and their feelings for their partners.[124] Assessment continues throughout treatment as new relevant information emerges.
[Figure caption and citation for the preceding image starts]: Algorithm of first-line therapy for women's sexual desire and arousal disorders (DHEA = dehydroepiandrosterone)Adapted from: Basson R, Leiblum S, Brotto L, et al. Revised definitions of women's sexual dysfunction. J Sex Med. 2004 Jul;1(1):40-8; used with permission [Citation ends].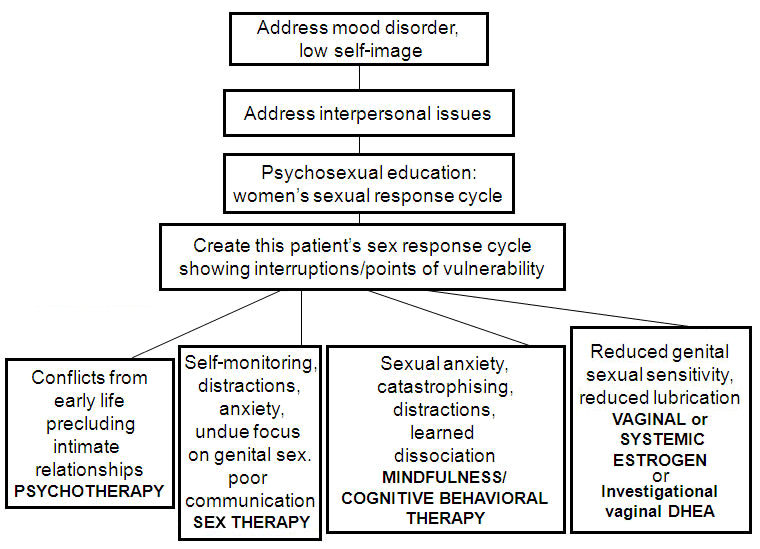 Treatment of persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) is not addressed here as there is limited research and its pathophysiology and treatment are not well understood.[13][12]
Treatment of persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) is not addressed here as there is limited research and its pathophysiology and treatment are not well understood.[13][12]
Initial management
Treat for any mood disorder.[25]
Counseling/referral for negative self-image and negative sexual self-image.[25]
Counseling/referral for interpersonal difficulties.[25]
Educating the couple regarding women's (and men's) sex response cycles. Explanation that suitable contexts and sexual stimuli to trigger desire are normal requirements.[125][126]
Use of cognitive therapy techniques to identify and challenge irrational thoughts and myths associated with a woman's sexual response, beliefs about herself, and misunderstandings about her partner's feelings/wishes. Use of behavior therapy specifically for lifelong female orgasmic disorder (FOD), teaching women how to reach climax via specific, structured, and graded exercises. Vibrostimulation may be suggested. Sex therapy in the form of sensate focus exchanges are used with the couple together and significantly target anticipatory and performance anxiety over sexual activity. They also guide the patient toward giving verbal and nonverbal feedback to the partner.[30]
Encouraging the woman to practice mindfulness techniques as a means of staying present and avoiding distractions, lessening self-criticism and evaluation, thereby allowing more intense sensations. She may be encouraged to practice mindfulness in nonsexual everyday life, then guided on how to specifically use this skill while being sexual.[127]
Addressing any untoward outcome (e.g., premature cessation of sexual activity with a male partner's ejaculation or continually striving for one or other partner's orgasm under the misunderstanding that it should always occur).
Considering use of pharmacologic adjuncts; drugs currently approved include vaginal estrogen for reduced congestion/lubrication. Vaginal dehydroepiandrosterone (DHEA) is approved in the US for treating dyspareunia related to menopause.
Types of psychological therapies
1. Psychoeducation
Given to all women treated for sexual concerns. It combines education and didactic information with elements of psychological therapy (e.g., cognitive and/or behavioral interventions).
Due to the widespread prevalence of myths about sexuality in women (e.g., the absence of desire preceding sexual activity denotes sexual dysfunction), psychoeducation is used during assessment and formulation to orient women to the context of their sexual complaints.
Emotion may be evoked via Socratic questioning, and the resultant irrational thoughts challenged by the clinician "in vivo" in order to replace them with more balanced evidence-based thoughts.
The use of bibliotherapy (self-help reading or videos) has been found to be helpful.[128][129]
For women with FOD, education on anatomy and physiology may be given along with normalizing the absence of coital orgasms, while simultaneously attending to affect and irrational thoughts.
Psychoeducation has been found to be effective for improving sexual complaints and distress among women with loss of genital swelling and lubrication secondary to gynecologic cancer.[130] A controlled study of mindfulness-based cognitive therapy showed benefit in sexual dysfunction subsequent to gynecologic cancer.[131] Study for the effectiveness of treatments for sexual dysfunction in women following cancer therapy has, however, been neglected.[132]
2. Cognitive behavioral therapy (CBT)
CBT is based on the premise that thoughts, feelings, and behavior are inextricably linked and that by changing one of these elements, the other two are influenced. It is a psychological treatment that aims to identify and challenge maladaptive (irrational) thoughts, reduce problematic behaviors, and, in turn, improve affect.
Meta-analyses have reported a large effect size from CBT on sexual desire, and also a moderate effect on improving sexual satisfaction.[133][134]
Among women with loss of sexual arousal, CBT may identify distractions that impede sexual arousal. For women with FOD, it may address emotions (e.g., fear of loss of control, vulnerability, perfectionism) that preclude orgasmic release.
3. Sex therapy
Sex therapy techniques originated with the work of Masters and Johnson; the most commonly used technique is sensate focus, a method of reducing performance anxiety and spectatoring (i.e., monitoring oneself during sexual activity).[135][136]
Sensate focus involves at least three stages during which couples will take turns to touch one another (initially not on the breast and genitals) and attempt to relax and attune to the sensations created. Both verbal and nonverbal feedback from the recipient is encouraged. With successive stages, more genital contact is permitted, along with mutual touching; however, intercourse is not allowed until the final stage. Original efficacy rates were extremely high, with a 5% relapse rate after 5 years. Subsequent efficacy rates reach approximately 65%.[137]
Sensate focus techniques remain useful for low desire (to increase women's awareness of effective stimuli), loss of arousal (to reduce anxiety and distractions), and FOD (to reduce anxiety and help women remain present with increasing arousal).
4. Psychodynamic therapies
Psychodynamic theories posit that sexual dysfunction is due to pathologic processes in personality development. Treatment therefore aims to improve a woman's ability to relate intimately to others by working through conflicts that lie at the heart of the sexual problem. Dream analysis, interpretations, and reclaiming memories of childhood may be included. Owing to the difficulty in manualizing psychodynamic treatment, there are no empiric studies for any form of female sexual dysfunction despite a rich clinical literature supporting these treatments among individuals and couples.
Systems therapy derives from family and marital therapy and is based on the premise that a system (i.e., a couple) is more than the sum of its parts. Systems therapy therefore focuses on different levels of interaction within the system (e.g., what are the shared symbols between the couple, what are the affect-regulated interactions, what are the sensate exchanges). While efficacy data on systems therapy for sexual dysfunction do not exist, it is viewed as being an excellent component to treatment for sexual dysfunction.[138][139]
Multielement treatments that incorporate cognitive and behavioral ingredients with systemic and psychodynamic approaches have also been described, and seem especially useful for difficult-to-treat couples.[140]
5. Mindfulness
Mindfulness involves repeated practice of exercises designed to keep one in the present (not judging what is experienced, monitoring but not following thoughts) and has been linked to reductions in stress and improved mood, energy, and wellbeing.
Mindfulness training has been shown to increase concordance between genital congestion from sexual stimulation and subjective arousal/excitement.[141]
Qualitative research suggests that the benefit from mindfulness may stem from lessening avoidance and from relinquishing sexual goals.[142]
Mindfulness significantly improved sexual response in women with self-reported sexual dysfunction subsequent to gynecologic cancer.[131] Study for the effectiveness of treatments for sexual dysfunction in women following cancer therapy has, however, been neglected.[132]
Mindfulness-based psychoeducational treatment may be especially beneficial for women with sexual desire and arousal disorders and who also have a history of sexual abuse.[125] In nondistressed couples, it significantly enhanced relationship happiness, reduced relationship stress, and improved stress-coping efficacy.[143]
6. Mindfulness-based cognitive therapy (MBCT)
MBCT can help to identify maladaptive and exaggerated thoughts that are viewed as "mental events," "what the brain does," and not necessarily true. Unlike CBT, which seeks to change such elements, encouragement is given to just let them exist for now without following or believing them. Benefit for women with sexual interest/arousal disorder (SIAD) as compared with detailed assessment and wait list has been shown.[126][127][144][145]
Adjunctive therapies
Vaginal estrogen:
Indicated if loss of genital arousal is due to vulvovaginal atrophy. Any vaginal formulation is appropriate (e.g., estradiol vaginal tablet, vaginal ring, or vaginal cream).[146]
However, it should be noted that high-dose estradiol cream should be limited to a single treatment period of up to 4 weeks due to the risk of adverse effects usually associated with systemic (oral or transdermal) hormone replacement therapy, and other vaginal estrogen formulations may be preferred.[147]
There is no approval for use of vaginal estrogen in women with previous oestrogen-dependent breast cancer; each woman must be assessed individually.[30] The American College of Obstetrics and Gynecologists advise that in these patients it should be reserved for those who are unresponsive to nonhormonal remedies.[148]
Systemic estrogen:
Only indicated if loss of genital congestion is identified to be due to vulvovaginal atrophy and systemic oestrogen is preferred because of other menopausal health considerations. Meta-analysis suggests estrogens alone or in combination with progestogens can allow a small to moderate improvement in sexual function, but mostly in pain, but not in unselected post-menopausal women.[149]
Vaginal DHEA:
Approved in the US for dyspareunia related to menopause. Preliminary evidence suggests vaginal DHEA benefits both atrophy (dryness and dyspareunia) and sexual response (orgasm intensity and desire/motivation).[46] Randomized controlled trials have also confirmed enhanced genital sexual sensitivity, as reflected by ease and intensity of orgasm, and increased desire, in postmenopausal women.[150]
Sex neutral antidepressants:
Investigational therapies
Testosterone:
In some European countries, a testosterone patch was approved on the basis of the first of two series of randomized controlled trials; however, at the request of the marketing authorization holder, the European Commission has withdrawn the marketing authorization for the testosterone patch.[155] The second series, using a gel formulation, showed no benefit beyond placebo.[156] Endogenous testosterone levels did not predict response to therapy.
Deficit of androgen activity has not been linked to low desire, and evidence on the safety and efficacy of testosterone use in women is mixed.[11][88][89] Careful evaluation found similar levels of serum testosterone and total androgen metabolites in women with low desire and arousal and in healthy controls.[11][157] In contrast, a number of studies report low DHEA levels in women with hypoactive sexual desire disorder (HSDD).[52]
One systematic review found a lack of sufficient evidence that testosterone treatment is efficacious and safe for treating low libido in premenopausal women. Although evidence suggested that higher doses may be more effective, the risks were hirsutism, clitoral enlargement, deepening of voice, and hair loss.[158]
One 2019 systematic review found a consistent benefit from testosterone treatment for naturally and surgically menopausal women, regardless of estrogen status. However, the clinical meaningfulness of the statistically significant benefit identified is much debated, and the lack of long-term safety data remains unaddressed.[90]
High endogenous levels of testosterone are associated with an increased risk of cardiovascular disease, breast cancer, and metabolic syndrome. Research including 2834 menopausal women found both a higher level of testosterone and a higher testosterone/estradiol ratio to be associated with increased cardiovascular and coronary heart disease.[159]
Despite this, some clinicians consider investigational testosterone supplementation when personal and interpersonal psychological issues and contextual factors are thought not to be etiologically important, and marked androgen deficiency is plausible. Such deficiency may arise from long term use of systemic corticosteroids, pituitary disease, or comorbid adrenal disease and premature ovarian failure.
In 2019, the Global Consensus Position Statement on the Use of Testosterone Therapy for Women recommended that investigational testosterone supplementation could be considered for fully informed and fully screened post-menopausal women with low sexual desire, and this recommendation now appears number of guidelines.[89][160][161][162] A time-limited trial of 3-6 months is recommended. Treatment should be stopped if there is no benefit by 6 months.[160] Important caveats include the level of supporting evidence, acceptable formulations, the need for full and ongoing informed consent and appropriate screening, and the need to disclose the lack of long term safety data (none available beyond 24 months of treatment).[160]
Strict laboratory and clinical monitoring is required to ensure testosterone levels are maintained within the premenopausal range, and patients require surveillance for signs of androgen excess.[160] However, accurate measurement of testosterone levels in women is challenging; liquid/gas chromatography and tandem mass spectrometry have high accuracy, but direct assays of total and free testosterone are highly unreliable in the female range.[160] Despite this, guidelines suggest direct assays can be used to exclude high baseline concentrations and supraphysiologic concentrations of testosterone during treatment when other methods are unavailable. Testosterone levels should be measured at baseline and after 3-6 weeks of treatment.[89][160]
As part of an informed consent process, amongst other warnings and risk/benefit analyses, the clinician should discuss concerns of potential cardiovascular harm, potential unknown risk of breast and endometrial cancer, potential irreversibility of some sequelae of androgen excess, and unknown long term effects.[159][163] This is of significance given potential need for long term therapy. Clinicians may also review the Global Consensus Position Statement with their patient.[160]
According to the Global Consensus Position Statement, data are insufficient to make any recommendations regarding use of supplemental testosterone in premenopausal women for treatment of sexual dysfunction, or for any other symptom, condition, or disease prevention in any age group.[160]
Flibanserin:
Flibanserin, a serotonin 1A receptor agonist and a serotonin 2A receptor antagonist, is approved in the US for the treatment of HSDD despite the benefit being similar to placebo and a serious risk of harm.[164][165] For these reasons, it is rarely used and many clinicians, including the authors of this topic do not recommend it. Published literature reviews also concluded that the empiric evidence for efficacy was insufficient to recommend its use.[166][167]
It is contraindicated in hepatic impairment, and may cause hypotension and syncope, especially if combined with alcohol. Alcohol use is contraindicated with the following recommendations: women should not drink alcohol for at least two hours before taking the bedtime dose, and no alcohol should be consumed after taking the bedtime dose until at least the next morning. If alcohol has been consumed within 2 hours before the bedtime dose, the dose should be skipped. The dose is taken at bedtime to reduce the risk of adverse effects due to hypotension, syncope, and central nervous system depression. Other risks include somnolence, fatigue, potential carcinogenicity, and potentially harmful drug interactions.[164][168] Flibanserin interacts with oral contraceptives to increase the risk of adverse reactions and make it potentially impractical for use in premenopausal sexually active women. Flibanserin is available only through a restricted risk evaluation and mitigation strategy program due to these risks.
Bremelanotide:
Bremelanotide is a melanocortin receptor agonist that nonselectively activates several melanocortin receptor subtypes including MC1R (expressed on melanocytes) and MC4R (expressed on neurons), and is administered subcutaneously. Its approval was based on two unpublished studies in premenopausal women with HSDD. Published data suggest bremelanotide improved sexual desire by around 0.4 points compared to placebo (on a scale of 1.2 to 6.0).[167][169] Adverse effects include nausea (40%), flushing (20%), transient increases in blood pressure and decreases in heart rate occurring after each dose, injection-site reactions, headache, vomiting, and focal hyperpigmentation (reported in one third of women if taken for 16 consecutive days).[169][170]
Many clinicians, including the authors of this topic, do not recommend its use given the questionable clinical benefit, adverse effects, and lack of long-term safety data.[167]
Use of this content is subject to our disclaimer