Tests
1st tests to order
biopsy and histopathologic assessment
Test
Skin biopsy confirms the diagnosis and provides prognostic information. Any nontender cutaneous nodule with nonspecific morphology that is fast growing should be biopsied rather than monitored.[7]
A punch, incisional, or excisional biopsy should be performed on any suspicious lesion.[3][7][39][43] The most appropriate method will depend on the size and location of the tumor.
Immunohistochemistry (IHC) must be used in conjunction with hematoxylin and eosin (H&E) for confirmation of the diagnosis and to rule out histologic mimics (most notably, other small cell tumors, including basal cell carcinoma, small cell lung cancer, and small cell melanoma).[3][7][29][39][43]
Cytokeratin 20 (CK20) positivity and thyroid transcription factor (TTF-1) negativity is characteristic of MCC, although variations are reported. MCC also stains positive for epithelial markers AE1/AE3, and CAM5.2, and for neuroendocrine markers such as neuron-specific enolase (NSE), synaptophysin, CD56, and chromogranin A. MCPyV large T expression is a specific but not wholly sensitive marker.
In addition to TTF-1 negativity, MCC is negative for S-100 and HMB-45, cytokeratin 7, carcinoembryonic antigen (CEA), leukocyte common antigen, and lymphoma-specific lymphocytic markers.
[Figure caption and citation for the preceding image starts]: Histologic features of MCC from biopsy of a primary tumor. Image B shows small round blue cells and image C shows characteristic nuclei, finely granular and dusty “salt and pepper” chromatin, and abundant mitotic figuresMauzo SH et al. J Clin Pathol 2016; 69: 382-90; used with permission [Citation ends].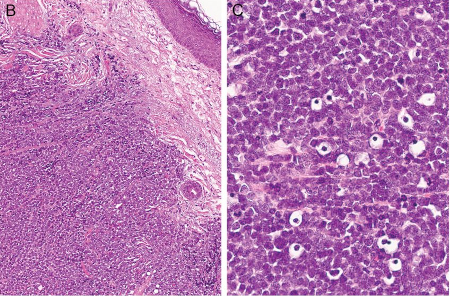
Result
H&E: dermally-based tumor of small round blue cells with hyperchromatic nuclei, "salt and pepper" chromatin pattern, and high mitotic activity; confirmatory IHC stains distinguish MCC from histopathologic mimics
dermoscopy
Test
Dermoscopy can be useful in raising suspicion of malignancy, although the dermoscopic pattern of MCC is nonspecific.[7] It can be particularly helpful for patients with multiple skin lesions as MCC can sometimes be contiguous to, or intermingled with, other skin cancers that can be more easily identified with dermoscopy.[19]
Result
may show prominent red or milky-red background color or smaller clods of milky-red areas, polymorphous vessels, and white areas
Tests to consider
lymph node ultrasound
Test
European guidelines recommend ultrasound of the regional lymph nodes in patients with clinical stage I or II disease to further evaluate for regional lymph node involvement.[19]
Result
may show regional disease
whole-body PET scan
Test
After confirmation of the diagnosis of MCC, imaging is recommended for most patients to evaluate for regional lymph node metastases and distant disease and for staging of the disease.[3][44]
Data indicate that whole-body fluorodeoxyglucose-positron emission tomography (FDG-PET) with fused axial imaging is the most reliable method for detecting occult metastatic MCC at baseline.[49][50] It is therefore recommended as the preferred cross-sectional imaging modality, if available, to assess local and distant disease.[3][19]
Result
may show metastases
CT scan with contrast chest/abdomen/pelvis (± neck)
brain MRI
Test
MRI of the brain (with or without contrast) is recommended if there is clinical suspicion of brain metastases (e.g., neurologic symptoms).[3]
Result
may show metastases
fine-needle aspiration or core biopsy
sentinel lymph node biopsy (SLNB)
Test
In patients presenting without detectable metastatic disease, use SLNB to identify subclinical metastatic disease in the regional nodal basin.[33][34][35][42][44][51]
SLNB should be performed prior to or at the time of excision of the primary tumor.[3][7]
SLNB has been demonstrated to detect lymph node spread in up to one third of patients who would have otherwise been staged as clinically node-negative.[52]
Result
may show metastases
Merkel cell polyomavirus serology
Test
Consider baseline serum testing of antibodies to MCPyV (AMERK test) as part of initial workup, if available.[3] If used, this should be undertaken within 3 months of initial treatment.
Results may guide subsequent surveillance; antibody titers can be monitored to help detect disease recurrence in those who are seropositive, whereas those who are seronegative have a worse prognosis and may need more intensive surveillance.[3]
In seropositive patients, consider re-testing every 3 months for surveillance, to detect recurrence and to help determine frequency of radiologic imaging.[53][54] Rise of antibody titer correlates with disease recurrence and fall of antibody titer correlates with treatment effect.[54] Two consecutive increasing titers of more than 20% have a positive predictive value for disease recurrence of 99%; decrease by 20% has a 99% negative predictive value for clinically detectable disease.[53][54]
Result
positive result indicates oncogenic transformation by MCPyV; post-treatment decreased oncoprotein antibody titer may indicate positive response; increasing titer may indicate disease recurrence
Emerging tests
circulating tumor DNA (ctDNA) analysis
Test
ctDNA is a blood test that measures circulating cell-free DNA from tumor cells, thereby offering molecular disease monitoring of different cancers, including MCC. ctDNA for MCC is an emerging area of interest for disease monitoring with few published data.[55][56]
Result
may detect minimal residual MCC ctDNA
Use of this content is subject to our disclaimer