Anaphylaxis
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
Look out for this icon: for treatment options that are affected, or added, as a result of your patient's comorbidities.
all patients: acute onset
1st line – cardiopulmonary assessment + supportive measures
cardiopulmonary assessment + supportive measures
Patients may present with symptoms that range in severity, but cardiac collapse and respiratory compromise cause the most urgent concern. Patients presenting with milder symptoms can rapidly deteriorate and should be closely monitored.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com
Regardless of severity, all patients with a diagnosis of anaphylaxis should be kept under observation until signs and symptoms have fully resolved.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com
Unless precluded by shortness of breath or vomiting, the patient should be placed in a supine position with legs elevated (shock or Trendelenburg position). This will augment venous return, and thereby increase preload and enhance cardiac output. Studies have shown that an upright position may contribute to a fatal outcome.[81]Pumphrey RS. Fatal posture in anaphylactic shock. J Allergy Clin Immunol. 2003 Aug;112(2):451-2. http://www.ncbi.nlm.nih.gov/pubmed/12897756?tool=bestpractice.com
intramuscular epinephrine (adrenaline)
Treatment recommended for ALL patients in selected patient group
Immediate administration of adequate doses of intramuscular epinephrine will decrease patient mortality and morbidity.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com [76]Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020 Oct 20;142(16 suppl 2):S366-468. https://www.doi.org/10.1161/CIR.0000000000000916 http://www.ncbi.nlm.nih.gov/pubmed/33081529?tool=bestpractice.com All patients with signs of a systemic reaction, especially hypotension, airway swelling, or difficulty breathing, should receive immediate intramuscular epinephrine in the anterolateral thigh.[76]Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020 Oct 20;142(16 suppl 2):S366-468. https://www.doi.org/10.1161/CIR.0000000000000916 http://www.ncbi.nlm.nih.gov/pubmed/33081529?tool=bestpractice.com [82]Dinakar, C. Anaphylaxis in children: current understanding and key issues in diagnosis and treatment. Curr Allergy Asthma Rep. 2012 Dec;12(6):641-9. http://link.springer.com/article/10.1007/s11882-012-0284-1/fulltext.html http://www.ncbi.nlm.nih.gov/pubmed/22815131?tool=bestpractice.com [83]Sicherer SH, Simons FE. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017 Mar;139(3):e20164006. http://pediatrics.aappublications.org/content/139/3/e20164006.long http://www.ncbi.nlm.nih.gov/pubmed/28193791?tool=bestpractice.com [84]Sheikh A, Simons FE, Barbour V, et al. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev. 2012 Aug 15;(8):CD008935. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008935.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/22895980?tool=bestpractice.com [85]Sheikh A, Shehata YA, Brown SG, et al. Adrenaline for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2009 Feb;64(2):204-12. http://onlinelibrary.wiley.com/doi/10.1111/j.1398-9995.2008.01926.x/full http://www.ncbi.nlm.nih.gov/pubmed/19178399?tool=bestpractice.com
This dose may be repeated every 5 to 15 minutes as needed.[6]Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report - second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006 Feb;117(2):391-7. http://www.jacionline.org/article/S0091-6749%2805%2902723-5/fulltext http://www.ncbi.nlm.nih.gov/pubmed/16461139?tool=bestpractice.com [12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com [76]Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020 Oct 20;142(16 suppl 2):S366-468. https://www.doi.org/10.1161/CIR.0000000000000916 http://www.ncbi.nlm.nih.gov/pubmed/33081529?tool=bestpractice.com [86]Cardona V, Ferré-Ybarz L, Guilarte M, et al. Safety of adrenaline use in anaphylaxis: a multicentre register. Int Arch Allergy Immunol. 2017;173(3):171-7. http://www.ncbi.nlm.nih.gov/pubmed/28793302?tool=bestpractice.com The anterolateral thigh is superior over intramuscular administration in the deltoid or subcutaneous injection.[87]Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001 Nov;108(5):871-3. http://www.ncbi.nlm.nih.gov/pubmed/11692118?tool=bestpractice.com [88]Simons FE, Roberts JR, Gu X, et al. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 1998 Jan;101(1 Pt 1):33-7. http://www.ncbi.nlm.nih.gov/pubmed/9449498?tool=bestpractice.com
It is usual practice to provide a prescription for two epinephrine auto-injectors after any episode of anaphylaxis due to the risk of severe anaphylaxis or a biphasic recurrence requiring multiple doses, although it may be reasonable to adopt a risk-stratified approach.[55]Golden DBK, Wang J, Waserman S, et al. Anaphylaxis: a 2023 practice parameter update. Ann Allergy Asthma Immunol. 2024 Feb;132(2):124-76. https://www.annallergy.org/article/S1081-1206(23)01304-2/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38108678?tool=bestpractice.com [90]Medicines and Healthcare products Regulatory Agency. Guidance on adrenaline auto-injectors (AAIs). Jun 2023 [internet publication]. https://www.gov.uk/government/publications/adrenaline-auto-injectors-aais-safety-campaign/adrenaline-auto-injectors-aais The patient or caregiver should carry both at all times and be familiar with their use.[83]Sicherer SH, Simons FE. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017 Mar;139(3):e20164006. http://pediatrics.aappublications.org/content/139/3/e20164006.long http://www.ncbi.nlm.nih.gov/pubmed/28193791?tool=bestpractice.com For children at risk of anaphylaxis, the epinephrine auto-injectors should be prescribed in conjunction with a personalized written emergency plan.[83]Sicherer SH, Simons FE. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017 Mar;139(3):e20164006. http://pediatrics.aappublications.org/content/139/3/e20164006.long http://www.ncbi.nlm.nih.gov/pubmed/28193791?tool=bestpractice.com [91]Wang J, Sicherer SH. Guidance on completing a written allergy and anaphylaxis emergency plan. Pediatrics. 2017 Mar;139(3):e20164005. http://pediatrics.aappublications.org/content/139/3/e20164005.long http://www.ncbi.nlm.nih.gov/pubmed/28193793?tool=bestpractice.com American Academy of Pediatrics: allergy and anaphylaxis emergency plan Opens in new window
Primary options
epinephrine (adrenaline): children: 0.01 mg/kg (as a 1:1000 solution) intramuscularly every 5-15 minutes, maximum 0.3 mg/dose; adults: 0.3 to 0.5 mg (as a 1:1000 solution) intramuscularly every 5-15 minutes
These drug options and doses relate to a patient with no comorbidities.
Primary options
epinephrine (adrenaline): children: 0.01 mg/kg (as a 1:1000 solution) intramuscularly every 5-15 minutes, maximum 0.3 mg/dose; adults: 0.3 to 0.5 mg (as a 1:1000 solution) intramuscularly every 5-15 minutes
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
epinephrine (adrenaline)
assess and secure airway
Treatment recommended for ALL patients in selected patient group
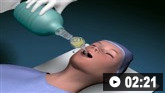
As even minor airway compromise can quickly progress to complete airway occlusion, immediate airway assessment and support is critical. If possible, airway assessment and management should be performed by a skilled anesthesiologist or emergency physician. Early prophylactic intubation or even cricothyrotomy may be necessary, especially if there is inspiratory stridor or evidence of worsening oral swelling (e.g., tongue, lip, or uvular swelling).[76]Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020 Oct 20;142(16 suppl 2):S366-468. https://www.doi.org/10.1161/CIR.0000000000000916 http://www.ncbi.nlm.nih.gov/pubmed/33081529?tool=bestpractice.com [80]Krishnaswamy G. Critical care management of the patient with anaphylaxis: a concise definitive review. Crit Care Med. 2021 May 1;49(5):838-57. https://www.doi.org/10.1097/CCM.0000000000004893 http://www.ncbi.nlm.nih.gov/pubmed/33653974?tool=bestpractice.com Inadequate respiratory efforts may indicate the need for ventilatory support.
How to insert a tracheal tube in an adult using a laryngoscope.
How to use bag-valve-mask apparatus to deliver ventilatory support to adults. Video demonstrates the two-person technique.
oxygen
Treatment recommended for ALL patients in selected patient group
Supplemental oxygen should be considered for all patients with anaphylaxis regardless of their respiratory status, and must be administered to any patient with respiratory or cardiovascular compromise and to those who do not respond to initial treatment with epinephrine (adrenaline).[53]Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020 Oct 30;13(10):100472. https://www.worldallergyorganizationjournal.org/article/S1939-4551(20)30375-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/33204386?tool=bestpractice.com [80]Krishnaswamy G. Critical care management of the patient with anaphylaxis: a concise definitive review. Crit Care Med. 2021 May 1;49(5):838-57. https://www.doi.org/10.1097/CCM.0000000000004893 http://www.ncbi.nlm.nih.gov/pubmed/33653974?tool=bestpractice.com
Breathing should be monitored by continuous oxygen saturation or blood gas determination. Inadequate respiratory efforts may indicate the need for ventilatory support.
intravenous fluids
Treatment recommended for ALL patients in selected patient group
Establish intravenous access.[53]Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020 Oct 30;13(10):100472. https://www.worldallergyorganizationjournal.org/article/S1939-4551(20)30375-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/33204386?tool=bestpractice.com Aggressive intravenous fluid replacement may be required (e.g., for patients with ongoing cardiovascular instability after epinephrine [adrenaline]), due to the intravascular volume redistribution into venous capacitance vessels and the interstitial tissue. The human vascular system consists of a high-pressure small-volume arterial system and a large low-pressure venous reservoir that expands in anaphylaxis, absorbing much of the circulating blood volume.
To make up for this intravascular fluid loss, adults should be administered a 20 mL/kg bolus (approximately 1 L to 2 L) of crystalloid fluid (e.g., normal saline) intravenously.[53]Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020 Oct 30;13(10):100472. https://www.worldallergyorganizationjournal.org/article/S1939-4551(20)30375-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/33204386?tool=bestpractice.com
Children should also receive a fluid bolus; see Volume depletion in children.
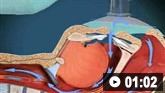
How to insert a peripheral intravascular catheter into the dorsum of the hand.
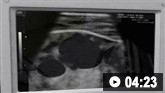
Ultrasound-guided insertion of a non-tunnelled central venous catheter (CVC) into the right internal jugular vein using the Seldinger insertion technique.
CPR + intravenous epinephrine (adrenaline)
Treatment recommended for ALL patients in selected patient group
If no pulse or breathing is present, CPR and advanced cardiac life support measures with intubation and ventilation are indicated. High-dose intravenous epinephrine is given.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com
How to insert a tracheal tube in an adult using a laryngoscope.
How to use bag-valve-mask apparatus to deliver ventilatory support to adults. Video demonstrates the two-person technique.
Primary options
epinephrine (adrenaline): children: 0.01 mg/kg (as a 1:10,000 solution) intravenously every 3-5 minutes, maximum 1 mg/dose; adults: 1 mg (as a 1:10,000 solution) intravenously every 3-5 minutes
These drug options and doses relate to a patient with no comorbidities.
Primary options
epinephrine (adrenaline): children: 0.01 mg/kg (as a 1:10,000 solution) intravenously every 3-5 minutes, maximum 1 mg/dose; adults: 1 mg (as a 1:10,000 solution) intravenously every 3-5 minutes
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
epinephrine (adrenaline)
intravenous epinephrine (adrenaline)
Treatment recommended for ALL patients in selected patient group
Intravenous epinephrine is administered to patients in cardiopulmonary arrest, and to profoundly hypotensive patients who have not responded to intravenous fluids and several doses of intramuscular epinephrine.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com [96]Muraro A, Tropeano A, Giovannini M. Allergen immunotherapy for food allergy: eidence and outlook. Allergol Select. 2022 Nov 21:6:285-92. https://pmc.ncbi.nlm.nih.gov/articles/PMC9707367 http://www.ncbi.nlm.nih.gov/pubmed/36457723?tool=bestpractice.com
Continuous intravenous infusion of epinephrine, titrated to effect, is reserved for experienced practitioners. No intravenous dose regimen is universally recognized.
Primary options
epinephrine (adrenaline): children: 0.01 mg/kg (as a 1:10,000 solution) intravenously every 5 minutes, maximum 0.3 mg/dose; adults: 0.1 mg (as a 1:10,000 solution) intravenously every 5 minutes
These drug options and doses relate to a patient with no comorbidities.
Primary options
epinephrine (adrenaline): children: 0.01 mg/kg (as a 1:10,000 solution) intravenously every 5 minutes, maximum 0.3 mg/dose; adults: 0.1 mg (as a 1:10,000 solution) intravenously every 5 minutes
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
epinephrine (adrenaline)
intravenous glucagon
Treatment recommended for SOME patients in selected patient group
In patients prescribed beta-blockers for coronary artery disease (CAD), both the medication and the underlying comorbidity complicate the treatment of severe anaphylaxis. The beta-blocker counteracts epinephrine (adrenaline) by limiting heart rate and compromising cardiac output. CAD limits cardiac reserve, which might compound the hypotension occurring due to the release of vasoactive mediators. The stresses of hypotension, tachycardia, and endogenous or iatrogenic adrenergic agents may cause myocardial ischemia by reducing perfusion during diastole. Glucagon may be used to overcome beta blockade, but the resulting tachycardia can be detrimental in patients with severe CAD.[6]Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report - second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006 Feb;117(2):391-7. http://www.jacionline.org/article/S0091-6749%2805%2902723-5/fulltext http://www.ncbi.nlm.nih.gov/pubmed/16461139?tool=bestpractice.com [55]Golden DBK, Wang J, Waserman S, et al. Anaphylaxis: a 2023 practice parameter update. Ann Allergy Asthma Immunol. 2024 Feb;132(2):124-76. https://www.annallergy.org/article/S1081-1206(23)01304-2/fulltext http://www.ncbi.nlm.nih.gov/pubmed/38108678?tool=bestpractice.com Therefore, early consultation of a cardiologist may be considered.
Primary options
glucagon: children: 0.02 to 0.03 mg/kg (maximum 1 mg/dose) intravenously initially, followed by 5-15 micrograms/minute infusion, titrate according to response; adults: 1-5 mg intravenously initially, followed by 5-15 micrograms/minute infusion, titrate according to response
More glucagonDose regimens may vary; consult your local drug information source for further guidance.
These drug options and doses relate to a patient with no comorbidities.
Primary options
glucagon: children: 0.02 to 0.03 mg/kg (maximum 1 mg/dose) intravenously initially, followed by 5-15 micrograms/minute infusion, titrate according to response; adults: 1-5 mg intravenously initially, followed by 5-15 micrograms/minute infusion, titrate according to response
More glucagonDose regimens may vary; consult your local drug information source for further guidance.
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
glucagon
inhaled beta-2 agonist
Treatment recommended for ALL patients in selected patient group
Persistent respiratory symptoms after the administration of epinephrine (adrenaline) may benefit from inhaled beta-2 agonists; should not be used as an alternative to the repeated administration of intramuscular epinephrine.[53]Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020 Oct 30;13(10):100472. https://www.worldallergyorganizationjournal.org/article/S1939-4551(20)30375-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/33204386?tool=bestpractice.com
The beta-2 agonist also has crossover activity at the beta-1 receptor and may therefore cause tachycardia and hypertension. Repeat dosing is limited by these adverse effects.
Primary options
albuterol inhaled: children: 0.15 mg/kg nebulized every 20 minutes for 3 doses, then 0.15 to 0.3 mg/kg every 1-4 hours when required; adults: 1.25 to 5 mg nebulized every 20 minutes for 3 doses, then every 1-4 hours when required
These drug options and doses relate to a patient with no comorbidities.
Primary options
albuterol inhaled: children: 0.15 mg/kg nebulized every 20 minutes for 3 doses, then 0.15 to 0.3 mg/kg every 1-4 hours when required; adults: 1.25 to 5 mg nebulized every 20 minutes for 3 doses, then every 1-4 hours when required
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
albuterol inhaled
H1 antagonist + H2 antagonist
Treatment recommended for SOME patients in selected patient group
Use of H1 and H2 antagonists is limited to relief of itching, hives, and rhinorrhea. Their use should never delay or replace the use of intramuscular epinephrine (adrenaline).[92]Sheikh A, Ten Broek V, Brown SG, et al. H1-antihistamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2007 Aug;62(8):830-7. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1398-9995.2007.01435.x http://www.ncbi.nlm.nih.gov/pubmed/17620060?tool=bestpractice.com [93]Andreae DA, Andreae MH. Should antihistamines be used to treat anaphylaxis? BMJ. 2009 Jul 10;339:b2489. http://www.ncbi.nlm.nih.gov/pubmed/19592404?tool=bestpractice.com Most antihistamines can be given intravenously, intramuscularly, or orally. In perioperative anaphylaxis, no evidence for harm in the administration of antihistamines was reported in a large UK audit.[27]Royal College of Anaesthetists. Anaesthesia, surgery and life-threatening allergic reactions. Report and findings of the Royal College of Anaesthetists’ 6th National Audit Project: perioperative anaphylaxis. May 2018 [internet publication]. https://www.rcoa.ac.uk/nap6-perioperative-anaphylaxis Oral administration may be sufficient for very mild allergic reactions but not anaphylaxis.
Primary options
diphenhydramine: children: 1-2 mg/kg intravenously/intramuscularly; adults: 25-50 mg intravenously/intramuscularly
and
cimetidine: children: consult specialist for guidance on dose; adults: 300 mg intravenously as a single dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
diphenhydramine: children: 1-2 mg/kg intravenously/intramuscularly; adults: 25-50 mg intravenously/intramuscularly
and
cimetidine: children: consult specialist for guidance on dose; adults: 300 mg intravenously as a single dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
diphenhydramine
and
cimetidine
corticosteroids
Treatment recommended for SOME patients in selected patient group
Corticosteroids may be prescribed as adjunctive therapy after the administration of epinephrine (adrenaline).[1]LoVerde D, Iweala OI, Eginli A, et al. Anaphylaxis. Chest. 2017 Aug 8;153(2):528-43. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6026262 http://www.ncbi.nlm.nih.gov/pubmed/28800865?tool=bestpractice.com [12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com Corticosteroids may decrease the risk of symptoms associated with anaphylaxis, including urticaria. Guidelines published in 2020 advise against administering corticosteroids to prevent biphasic anaphylaxis. This is based on limited evidence suggesting that there is no clear benefit in terms of risk reduction.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com
Although corticosteroids are not routinely recommended for the acute management of anaphylaxis, they may be considered after the administration of epinephrine for refractory reactions or where an acute asthma exacerbation may have contributed to the severity of anaphylaxis.[94]Resuscitation Council UK. Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Primary options
methylprednisolone: children and adults: 1-2 mg/kg/day intravenously
OR
prednisone: children and adults: 0.5 to 1 mg/kg/day orally
These drug options and doses relate to a patient with no comorbidities.
Primary options
methylprednisolone: children and adults: 1-2 mg/kg/day intravenously
OR
prednisone: children and adults: 0.5 to 1 mg/kg/day orally
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
methylprednisolone
OR
prednisone
patients with identified allergen
immunotherapy
Venom immunotherapy may be recommended for prevention of systemic reactions in patients with a history of anaphylaxis subsequent to insect sting.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123.
https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com
[74]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77.
https://www.doi.org/10.1111/all.15032
http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
[  ]
Is there randomized controlled trial evidence to support the use of venom immunotherapy to prevent allergic reactions to insect stings?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.148/fullShow me the answer The treatment is highly effective at preventing these systemic reactions.[95]Boyle RJ, Elremeli M, Hockenhull J, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012 Oct 17;(10):CD008838.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008838.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/23076950?tool=bestpractice.com
Venom immunotherapy increases the risk of adverse systemic reactions during treatment.[95]Boyle RJ, Elremeli M, Hockenhull J, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012 Oct 17;(10):CD008838.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008838.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/23076950?tool=bestpractice.com
]
Is there randomized controlled trial evidence to support the use of venom immunotherapy to prevent allergic reactions to insect stings?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.148/fullShow me the answer The treatment is highly effective at preventing these systemic reactions.[95]Boyle RJ, Elremeli M, Hockenhull J, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012 Oct 17;(10):CD008838.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008838.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/23076950?tool=bestpractice.com
Venom immunotherapy increases the risk of adverse systemic reactions during treatment.[95]Boyle RJ, Elremeli M, Hockenhull J, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012 Oct 17;(10):CD008838.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008838.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/23076950?tool=bestpractice.com
Avoidance of food allergens remains the preventive mainstay of food-induced anaphylaxis.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com [96]Muraro A, Tropeano A, Giovannini M. Allergen immunotherapy for food allergy: eidence and outlook. Allergol Select. 2022 Nov 21:6:285-92. https://pmc.ncbi.nlm.nih.gov/articles/PMC9707367 http://www.ncbi.nlm.nih.gov/pubmed/36457723?tool=bestpractice.com Subcutaneous, epicutaneous (via absorbed patch), oral, and sublingual immunotherapy routes have been assessed. Studies suggest that while treatment may lead to desensitization, few patients attain tolerance.[96]Muraro A, Tropeano A, Giovannini M. Allergen immunotherapy for food allergy: eidence and outlook. Allergol Select. 2022 Nov 21:6:285-92. https://pmc.ncbi.nlm.nih.gov/articles/PMC9707367 http://www.ncbi.nlm.nih.gov/pubmed/36457723?tool=bestpractice.com Food-allergy-specific immunotherapy continues to be researched; it has historically been associated with adverse reactions, including anaphylaxis.[96]Muraro A, Tropeano A, Giovannini M. Allergen immunotherapy for food allergy: eidence and outlook. Allergol Select. 2022 Nov 21:6:285-92. https://pmc.ncbi.nlm.nih.gov/articles/PMC9707367 http://www.ncbi.nlm.nih.gov/pubmed/36457723?tool=bestpractice.com Currently, oral immunotherapy peanut allergy is approved for use under specialist supervision by the Food and Drug Administration (FDA) for the mitigation of allergic reactions including anaphylaxis, which may occur with exposure to peanuts.[97]Borne GE, Daniel CP, Wagner MJ, et al. Palforzia for peanut allergy: a narrative review and update on a novel immunotherapy. Cureus. 2023 Dec 13;15(12):e50485. https://www.cureus.com/articles/207548-palforzia-for-peanut-allergy-a-narrative-review-and-update-on-a-novel-immunotherapy#! http://www.ncbi.nlm.nih.gov/pubmed/38222206?tool=bestpractice.com See Food allergy for further information.
Drug desensitization may be considered to induce temporary drug tolerance in patients with immunoglobulin E-mediated drug-induced anaphylaxis who require the causal drug, and where there is no effective alternative option.[12]Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-123. https://www.jacionline.org/article/S0091-6749(20)30105-6/fulltext http://www.ncbi.nlm.nih.gov/pubmed/32001253?tool=bestpractice.com [74]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77. https://www.doi.org/10.1111/all.15032 http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com Drug desensitization should be performed by experienced clinicians in an appropriate setting.

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer