Sepsis in adults
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
Look out for this icon: for treatment options that are affected, or added, as a result of your patient's comorbidities.
presumed or confirmed sepsis
broad-spectrum empiric intravenous antibiotics
Broad-spectrum empiric intravenous antibiotics should be given before a pathogen is identified.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [129]Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009 Nov;136(5):1237-48. http://www.ncbi.nlm.nih.gov/pubmed/19696123?tool=bestpractice.com [130]Kumar A, Roberts D, Wood KE, et al. Duration of hypotension prior to initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589-96. http://www.ncbi.nlm.nih.gov/pubmed/16625125?tool=bestpractice.com They should be commenced as soon as sepsis is suspected, preferably after cultures have been taken.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [10]National Institute for Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. March 2024 [internet publication]. https://www.nice.org.uk/guidance/ng51 The timely delivery of appropriate antibiotics is of critical importance in maximizing chances of survival.[69]Daniels R, Nutbeam I, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011 Jun;28(6):507-12. http://www.ncbi.nlm.nih.gov/pubmed/21036796?tool=bestpractice.com [132]Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010 Apr;38(4):1045-53. http://www.ncbi.nlm.nih.gov/pubmed/20048677?tool=bestpractice.com [133]Johnston AN, Park J, Doi SA, et al. Effect of immediate administration of antibiotics in patients with sepsis in tertiary care: a systematic review and meta-analysis. Clin Ther. 2017 Jan;39(1):190-202;e6. http://www.ncbi.nlm.nih.gov/pubmed/28062114?tool=bestpractice.com [134]National Institute for Health Research. NIHR Alert: giving immediate antibiotics reduces deaths from sepsis. Apr 2017 [internet publication]. https://evidence.nihr.ac.uk/alert/giving-immediate-antibiotics-reduces-deaths-from-sepsis [135]Sherwin R, Winters ME, Vilke GM, et al. Does early and appropriate antibiotic administration improve mortality in emergency department patients with severe sepsis or septic shock? J Emerg Med. 2017 Oct;53(4):588-95. http://www.ncbi.nlm.nih.gov/pubmed/28916120?tool=bestpractice.com [136]Baghdadi JD, Brook RH, Uslan DZ, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020 May 1;180(5):707-16. http://www.ncbi.nlm.nih.gov/pubmed/32250412?tool=bestpractice.com [137]Bisarya R, Song X, Salle J, et al. Antibiotic timing and progression to septic shock among patients in the ED with suspected infection. Chest. 2022 Jan;161(1):112-20. http://www.ncbi.nlm.nih.gov/pubmed/34186038?tool=bestpractice.com [138]Bassetti M, Rello J, Blasi F, et al. Systematic review of the impact of appropriate versus inappropriate initial antibiotic therapy on outcomes of patients with severe bacterial infections. Int J Antimicrob Agents. 2020 Dec;56(6):106184. http://www.ncbi.nlm.nih.gov/pubmed/33045353?tool=bestpractice.com
Antibiotics should be targeted to the presumed site of infection. If there is no clinical evidence to suggest a specific site of infection and sepsis is still suspected, empiric broad-spectrum antibiotics should still be given.
Once culture and sensitivity results are known, antibiotics should be narrowed if it is possible to do so.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [141]Paul M, Dickstein Y, Raz-Pasteur A. Antibiotic de-escalation for bloodstream infections and pneumonia: systematic review and meta-analysis. Clin Microbiol Infect. 2016 Dec;22(12):960-7. http://www.ncbi.nlm.nih.gov/pubmed/27283148?tool=bestpractice.com
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com [129]Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009 Nov;136(5):1237-48. http://www.ncbi.nlm.nih.gov/pubmed/19696123?tool=bestpractice.com
One systematic review found that prolonged infusion of antipseudomonal beta-lactam antibiotics over at least 3 hours (rather than a bolus or within 60 minutes) reduced mortality by up to 30% in patients with sepsis in the intensive care.[139]Vardakas KZ, Voulgaris GL, Maliaros A, et al. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018 Jan;18(1):108-20. http://www.ncbi.nlm.nih.gov/pubmed/29102324?tool=bestpractice.com
Cultures should be repeated (e.g., at 6-hour to 8-hour intervals) if there are persistent or repeated fever spikes, or there is the identification of a new site of infection.
See below for specific choice of antibiotic regimens based on source of infection.
fluid resuscitation
Treatment recommended for ALL patients in selected patient group
Early fluid resuscitation in sepsis-induced hypoperfusion is needed and admission to the high dependency unit or intensive care setting should be considered.[65]Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-77.
https://www.nejm.org/doi/10.1056/NEJMoa010307
http://www.ncbi.nlm.nih.gov/pubmed/11794169?tool=bestpractice.com
[166]Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients; 2004 update. Crit Care Med. 2004 Sep;32(9):1928-48.
http://www.ncbi.nlm.nih.gov/pubmed/15343024?tool=bestpractice.com
At least 30 mL/kg body weight of crystalloid should be given within the first 3 hours, commenced within 1 hour of sepsis being suspected.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx
http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
[127]Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign bundle: 2018 update. Intensive Care Med. 2018 Jun;44(6):925-8.
https://link.springer.com/article/10.1007%2Fs00134-018-5085-0
http://www.ncbi.nlm.nih.gov/pubmed/29675566?tool=bestpractice.com
However, optimal targets for fluid resuscitation should be individualized in certain patients, such as those with heart failure or chronic kidney disease, due to the risk of volume overload.[150]Weng J, Xu Z, Song J, et al. Optimal fluid resuscitation targets in septic patients with acutely decompensated heart failure. BMC Med. 2024 Oct 24;22(1):492.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11520127
http://www.ncbi.nlm.nih.gov/pubmed/39448976?tool=bestpractice.com
[151]Jorda A, Douglas IS, Staudinger T, et al. Fluid management for sepsis-induced hypotension in patients with advanced chronic kidney disease: a secondary analysis of the CLOVERS trial. Crit Care. 2024 Jul 11;28(1):231.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11238412
http://www.ncbi.nlm.nih.gov/pubmed/38992663?tool=bestpractice.com
[152]Rajdev K, Leifer L, Sandhu G, et al. Aggressive versus conservative fluid resuscitation in septic hemodialysis patients. Am J Emerg Med. 2021 Aug;46:416-9.
http://www.ncbi.nlm.nih.gov/pubmed/33129646?tool=bestpractice.com
Additional fluid may be required, but this should be guided by thorough clinical assessment of the patient's hemodynamic status.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx
http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
Repeated fluid boluses of crystalloid (typical volume 500 mL) given over 5-30 minutes may be effective in correcting hypotension secondary to hypovolemia. Boluses of a colloid solution (typical volume 250-300 mL) may be used as an alternative, but no evidence supports the use of colloids over other fluids such as crystalloids or albumin solution.[153]Choi PTL, Yip G, Quinonez LG, et al. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999 Jan;27(1):200-10.
http://www.ncbi.nlm.nih.gov/pubmed/9934917?tool=bestpractice.com
[154]Finfer S, Bellomo R, Boyce N, et al; the SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004 May 27;350(22):2247-56.
https://www.nejm.org/doi/full/10.1056/NEJMoa040232
http://www.ncbi.nlm.nih.gov/pubmed/15163774?tool=bestpractice.com
[155]Lewis SR, Pritchard MW, Evans DJ, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018 Aug 3;(8):CD000567.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000567.pub7/full
http://www.ncbi.nlm.nih.gov/pubmed/30073665?tool=bestpractice.com
[156]Roberts I, Blackhall K, Alderson P, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2011 Nov 9;(11):CD001208.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001208.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/22071799?tool=bestpractice.com
[157]Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014 Sep 2;161(5):347-55.
http://www.ncbi.nlm.nih.gov/pubmed/25047428?tool=bestpractice.com
[  ]
How do colloids compare with crystalloids for fluid resuscitation in critically ill people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2307/fullShow me the answer
]
How do colloids compare with crystalloids for fluid resuscitation in critically ill people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2307/fullShow me the answer
Resuscitation with crystalloids requires more volume than colloids. Crystalloids and albumin should be given according to local protocols. Current evidence suggests that resuscitation using albumin-containing solutions is safe, but evidence of its efficacy is insufficient to recommend its use over crystalloids.[157]Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014 Sep 2;161(5):347-55. http://www.ncbi.nlm.nih.gov/pubmed/25047428?tool=bestpractice.com [158]Patel A, Laffan MA, Waheed U, et al. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ. 2014 Jul 22;349:g4561. [Erratum in: BMJ. 2014;349:g4850.] https://www.bmj.com/content/349/bmj.g4561 http://www.ncbi.nlm.nih.gov/pubmed/25099709?tool=bestpractice.com [159]Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011 Feb;39(2):386-91. http://www.ncbi.nlm.nih.gov/pubmed/21248514?tool=bestpractice.com Balanced crystalloids may be preferable to normal saline in critically sick patients in intensive care.[161]Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829-39. http://www.ncbi.nlm.nih.gov/pubmed/29485925?tool=bestpractice.com
The Surviving Sepsis Campaign advises against the use of solutions containing hydroxyethyl starch (HES) for patients with sepsis and septic shock.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com In July 2021, the US Food and Drug Administration (FDA) issued safety labeling changes for solutions containing HES stating that these products should not be used unless adequate alternative treatment is unavailable.[162]US Food and Drug Administration. Labeling changes on mortality, kidney injury, and excess bleeding with hydroxyethyl starch products. Jul 2021 [internet publication]. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products HES products are associated with adverse outcomes including kidney injury and death, particularly in critically ill patients and those with sepsis.[155]Lewis SR, Pritchard MW, Evans DJ, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018 Aug 3;(8):CD000567. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000567.pub7/full http://www.ncbi.nlm.nih.gov/pubmed/30073665?tool=bestpractice.com [163]Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013 Feb 20;309(7):678-88. https://jamanetwork.com/journals/jama/fullarticle/1653505 http://www.ncbi.nlm.nih.gov/pubmed/23423413?tool=bestpractice.com In view of the serious risks posed to these patient populations, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency in February 2022 recommended suspending HES solutions for infusion in Europe.[164]European Medicines Agency. PRAC recommends suspending hydroxyethyl-starch solutions for infusion from the market. Feb 2022 [internet publication]. https://www.ema.europa.eu/en/news/prac-recommends-suspending-hydroxyethyl-starch-solutions-infusion-market-0
Red cell or plasma transfusion may be considered to target specific deficiencies but should not be used for volume augmentation. Aggressive red cell transfusion has not been shown to improve outcomes.[165]Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014 Oct 9;371(15):1381-91. https://www.nejm.org/doi/full/10.1056/NEJMoa1406617 http://www.ncbi.nlm.nih.gov/pubmed/25270275?tool=bestpractice.com
Patients should be monitored closely for signs of fluid overload (e.g., pulmonary or systemic edema).[166]Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients; 2004 update. Crit Care Med. 2004 Sep;32(9):1928-48. http://www.ncbi.nlm.nih.gov/pubmed/15343024?tool=bestpractice.com [204]Cherpanath TG, Hirsch A, Geerts BF, et al. Predicting fluid responsiveness by passive leg raising: a systematic review and meta-analysis of 23 clinical trials. Crit Care Med. 2016 May;44(5):981-91. http://www.ncbi.nlm.nih.gov/pubmed/26741579?tool=bestpractice.com
oxygen
Treatment recommended for SOME patients in selected patient group
Administer oxygen, if indicated, to maintain target oxygen saturations greater than 94% (or 88% to 92% in people at risk of hypercapnic respiratory failure).[69]Daniels R, Nutbeam I, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011 Jun;28(6):507-12. http://www.ncbi.nlm.nih.gov/pubmed/21036796?tool=bestpractice.com
One systematic review of acutely sick adult patients, including patients with sepsis, reported increased mortality among those who received liberal oxygen supplementation compared with those who received conservative oxygen supplementation. A target SpO₂ range of 94% to 96% was suggested for patients with critical illness.[128]Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018 Apr 28;391(10131):1693-705. http://www.ncbi.nlm.nih.gov/pubmed/29726345?tool=bestpractice.com
Consider – standard supportive intensive care setting (ICU) care
standard supportive intensive care setting (ICU) care
Treatment recommended for SOME patients in selected patient group
All patients with sepsis should be considered for admission to the high dependency unit or ICU.[65]Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-77. https://www.nejm.org/doi/10.1056/NEJMoa010307 http://www.ncbi.nlm.nih.gov/pubmed/11794169?tool=bestpractice.com [166]Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients; 2004 update. Crit Care Med. 2004 Sep;32(9):1928-48. http://www.ncbi.nlm.nih.gov/pubmed/15343024?tool=bestpractice.com
General intensive care measures include stress ulcer prophylaxis with H2 antagonists or proton-pump inhibitors (in patients at risk of gastrointestinal bleeding), deep venous thrombosis prophylaxis (with heparin and compression stockings), enteral or parenteral nutrition, and glycemic control.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [182]Trzeciak S, Dellinger RP. Other supportive therapies in sepsis: an evidence-based review. Crit Care Med. 2004 Nov;32(suppl 11):S571-7. http://www.ncbi.nlm.nih.gov/pubmed/15542966?tool=bestpractice.com [183]Nguyen HB, Rivers EP, Abrahamian FM, et al; Emergency Department Sepsis Education Program and Strategies to Improve Survival (ED-SEPSIS) Working Group. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006 Jul;48(1):28-54. http://www.ncbi.nlm.nih.gov/pubmed/16781920?tool=bestpractice.com [184]Wang Y, Ye Z, Ge L, et al. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: systematic review and network meta-analysis. BMJ. 2020 Jan 6;368:l6744. https://www.bmj.com/content/368/bmj.l6744 http://www.ncbi.nlm.nih.gov/pubmed/31907166?tool=bestpractice.com The American Diabetes Association recommends a general glucose goal of 140-180 mg/dL in critically ill diabetic patients, preferably with use of an insulin infusion protocol.[170]American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of care in diabetes-2025. Diabetes Care. 2025 Jan 1;48(supplement_1):S321-34. https://www.doi.org/10.2337/dc25-S016 http://www.ncbi.nlm.nih.gov/pubmed/39651972?tool=bestpractice.com The Surviving Sepsis Campaign recommends the use of validated insulin infusion protocols targeting a blood glucose level of <180 mg/dL.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
Transfusion of packed cells may be required; a restrictive transfusion strategy using a target hemoglobin concentration of 7 g/dL is recommended.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [165]Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014 Oct 9;371(15):1381-91. https://www.nejm.org/doi/full/10.1056/NEJMoa1406617 http://www.ncbi.nlm.nih.gov/pubmed/25270275?tool=bestpractice.com [185]Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999 Feb 11;340(6):409-17. https://www.nejm.org/doi/full/10.1056/NEJM199902113400601 http://www.ncbi.nlm.nih.gov/pubmed/9971864?tool=bestpractice.com [186]Coz Yataco AO, Soghier I, Hébert PC, et al. Red blood cell transfusion in critically ill adults: an American College of Chest Physicians clinical practice guideline. Chest. 2025 Feb;167(2):477-89. https://www.doi.org/10.1016/j.chest.2024.09.016 http://www.ncbi.nlm.nih.gov/pubmed/39341492?tool=bestpractice.com A higher hemoglobin level may be necessary in certain circumstances (myocardial ischemia, severe hypoxemia, or acute hemorrhage).[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [186]Coz Yataco AO, Soghier I, Hébert PC, et al. Red blood cell transfusion in critically ill adults: an American College of Chest Physicians clinical practice guideline. Chest. 2025 Feb;167(2):477-89. https://www.doi.org/10.1016/j.chest.2024.09.016 http://www.ncbi.nlm.nih.gov/pubmed/39341492?tool=bestpractice.com In the initial resuscitative phase a higher hematocrit of ≥30% may be appropriate.[65]Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-77. https://www.nejm.org/doi/10.1056/NEJMoa010307 http://www.ncbi.nlm.nih.gov/pubmed/11794169?tool=bestpractice.com
Patients requiring prolonged ventilatory support should receive lung protective ventilation using minimal peak inspiratory pressures (<30 cmH₂O) to specifically limit pulmonary compromise.[187]Brower RG, Matthay MA, Morris A, et al; Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8. https://www.nejm.org/doi/full/10.1056/NEJM200005043421801 http://www.ncbi.nlm.nih.gov/pubmed/10793162?tool=bestpractice.com FiO₂ should be titrated to lowest effective levels to prevent oxygen toxicity and maintain central venous oxygen tension. One systematic review of acutely sick adult patients, including patients with sepsis, reported increased mortality among those who received liberal oxygen supplementation compared with those who received conservative oxygen supplementation. A target SpO₂ range of 94% to 96% was suggested for all patients with critical sickness.[128]Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018 Apr 28;391(10131):1693-705. http://www.ncbi.nlm.nih.gov/pubmed/29726345?tool=bestpractice.com
Bathing patients with chlorhexidine washes may reduce the risk of hospital-acquired infection.[180]Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013 Feb 7;368(6):533-42. https://www.nejm.org/doi/10.1056/NEJMoa1113849 http://www.ncbi.nlm.nih.gov/pubmed/23388005?tool=bestpractice.com Intravenous acetylcysteine as an adjuvant therapy in systemic inflammatory response syndrome (SIRS) and sepsis has been found to be ineffective, and its use is not recommended.[181]Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012 Sep 12;(9):CD006616. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006616.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/22972094?tool=bestpractice.com
For critically ill patients with fever, routine use of antipyretic medications should be avoided for the specific purpose of reducing the temperature.[82]O'Grady NP, Alexander E, Alhazzani W, et al. Society of Critical Care Medicine and the Infectious Diseases Society of America guidelines for evaluating new fever in adult patients in the ICU. Crit Care Med. 2023 Nov 1;51(11):1570-86. https://journals.lww.com/ccmjournal/fulltext/2023/11000/society_of_critical_care_medicine_and_the.13.aspx http://www.ncbi.nlm.nih.gov/pubmed/37902340?tool=bestpractice.com However, where reduction of fever is required for the comfort of the patient, using antipyretics over nonpharmacologic methods to reduce body temperature is recommended.[82]O'Grady NP, Alexander E, Alhazzani W, et al. Society of Critical Care Medicine and the Infectious Diseases Society of America guidelines for evaluating new fever in adult patients in the ICU. Crit Care Med. 2023 Nov 1;51(11):1570-86. https://journals.lww.com/ccmjournal/fulltext/2023/11000/society_of_critical_care_medicine_and_the.13.aspx http://www.ncbi.nlm.nih.gov/pubmed/37902340?tool=bestpractice.com
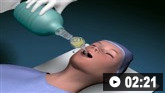
How to insert a tracheal tube in an adult using a laryngoscope.
How to use bag-valve-mask apparatus to deliver ventilatory support to adults. Video demonstrates the two-person technique.
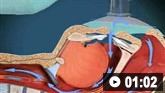
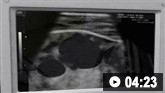
Ultrasound-guided insertion of a non-tunnelled central venous catheter (CVC) into the right internal jugular vein using the Seldinger insertion technique.
vasopressors
Treatment recommended for SOME patients in selected patient group
Patients unresponsive to fluid resuscitation may be treated with vasopressors.
Patients requiring vasoactive support (those with septic shock) are identified by a mean arterial pressure (MAP) <65 mmHg, persisting after adequate fluid resuscitation.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [70]Yealy DM, Mohr NM, Shapiro NI, et al. Early care of adults with suspected sepsis in the emergency department and out-of-hospital environment: a consensus-based task force Report. Ann Emerg Med. 2021 Jul;78(1):1-19. https://www.annemergmed.com/article/S0196-0644(21)00117-7/fulltext http://www.ncbi.nlm.nih.gov/pubmed/33840511?tool=bestpractice.com
The Surviving Sepsis Campaign recommends aiming for a MAP of 65 mmHg in patients with septic shock.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
Norepinephrine administered via a central venous catheter is the drug of choice as it increases MAP.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [171]Avni T, Lador A, Lev S, et al. Vasopressors for the treatment of septic shock: systematic review and meta-analysis. PLoS One. 2015 Aug 3;10(8):e0129305. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0129305 http://www.ncbi.nlm.nih.gov/pubmed/26237037?tool=bestpractice.com [172]Møller MH, Claudius C, Junttila E, et al. Scandinavian SSAI clinical practice guideline on choice of first-line vasopressor for patients with acute circulatory failure. Acta Anaesthesiol Scand. 2016 Nov;60(10):1347-66. https://onlinelibrary.wiley.com/doi/10.1111/aas.12780 http://www.ncbi.nlm.nih.gov/pubmed/27576362?tool=bestpractice.com Vasopressin can be added to norepinephrine to achieve the target MAP.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [70]Yealy DM, Mohr NM, Shapiro NI, et al. Early care of adults with suspected sepsis in the emergency department and out-of-hospital environment: a consensus-based task force Report. Ann Emerg Med. 2021 Jul;78(1):1-19. https://www.annemergmed.com/article/S0196-0644(21)00117-7/fulltext http://www.ncbi.nlm.nih.gov/pubmed/33840511?tool=bestpractice.com
Vasopressors may be started via a peripheral venous line if there is a delay in securing central venous access.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [70]Yealy DM, Mohr NM, Shapiro NI, et al. Early care of adults with suspected sepsis in the emergency department and out-of-hospital environment: a consensus-based task force Report. Ann Emerg Med. 2021 Jul;78(1):1-19. https://www.annemergmed.com/article/S0196-0644(21)00117-7/fulltext http://www.ncbi.nlm.nih.gov/pubmed/33840511?tool=bestpractice.com
Primary options
norepinephrine: 0.02 to 0.5 micrograms/kg/minute intravenously initially, titrate to effect
Secondary options
norepinephrine: 0.02 to 0.5 micrograms/kg/minute intravenously initially, titrate to effect
and
vasopressin: 0.01 to 0.03 units/minute intravenously initially, titrate to effect
These drug options and doses relate to a patient with no comorbidities.
Primary options
norepinephrine: 0.02 to 0.5 micrograms/kg/minute intravenously initially, titrate to effect
Secondary options
norepinephrine: 0.02 to 0.5 micrograms/kg/minute intravenously initially, titrate to effect
and
vasopressin: 0.01 to 0.03 units/minute intravenously initially, titrate to effect
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
norepinephrine
Secondary options
norepinephrine
and
vasopressin
inotropes
Treatment recommended for SOME patients in selected patient group
In patients with low cardiac output despite adequate fluid resuscitation, inotropes can be added. Selection of appropriate vasoactive agents should only take place under critical care supervision and may vary according to clinician preference and local practice guidelines.
Dobutamine is the first choice inotrope for patients with low cardiac output and adequate left ventricular filling pressure (or adequate fluid administration) and adequate mean arterial pressure.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com [166]Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients; 2004 update. Crit Care Med. 2004 Sep;32(9):1928-48. http://www.ncbi.nlm.nih.gov/pubmed/15343024?tool=bestpractice.com
Primary options
dobutamine: 0.5 to 1 microgram/kg/minute intravenously initially, followed by 2-20 micrograms/kg/minute
These drug options and doses relate to a patient with no comorbidities.
Primary options
dobutamine: 0.5 to 1 microgram/kg/minute intravenously initially, followed by 2-20 micrograms/kg/minute
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
dobutamine
corticosteroids
Treatment recommended for SOME patients in selected patient group
Evidence for giving corticosteroids to patients with sepsis or septic shock is mixed and guideline recommendations vary.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143.
https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx
http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
[70]Yealy DM, Mohr NM, Shapiro NI, et al. Early care of adults with suspected sepsis in the emergency department and out-of-hospital environment: a consensus-based task force Report. Ann Emerg Med. 2021 Jul;78(1):1-19.
https://www.annemergmed.com/article/S0196-0644(21)00117-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33840511?tool=bestpractice.com
[174]Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017 Dec;45(12):2078-88.
http://www.ncbi.nlm.nih.gov/pubmed/28938253?tool=bestpractice.com
[175]Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev. 2019 Dec 6;(12):CD002243.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002243.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/31808551?tool=bestpractice.com
[176]Kalil AC, Sun J. Low-dose steroids for septic shock and severe sepsis: the use of Bayesian statistics to resolve clinical trial controversies. Intensive Care Med. 2011 Mar;37(3):420-9.
http://www.ncbi.nlm.nih.gov/pubmed/21243334?tool=bestpractice.com
[177]Rygård SL, Butler E, Granholm A, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018 Jul;44(7):1003-16.
http://www.ncbi.nlm.nih.gov/pubmed/29761216?tool=bestpractice.com
[  ]
What are the effects of corticosteroids for people with sepsis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2908/fullShow me the answer[Evidence A]b93ad1b4-c270-4e77-9c38-1a72d7be08ceccaAWhat are the effects of corticosteroids for people with sepsis?
]
What are the effects of corticosteroids for people with sepsis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2908/fullShow me the answer[Evidence A]b93ad1b4-c270-4e77-9c38-1a72d7be08ceccaAWhat are the effects of corticosteroids for people with sepsis?
One clinical practice guideline, informed by meta-analysis, reported that corticosteroids may reduce mortality (by approximately 2%) and increase the risk of neuromuscular weakness, but evidence is not definitive.[178]Lamontagne F, Rochwerg B, Lytvyn L, et al. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018 Aug 10;362:k3284. https://www.bmj.com/content/362/bmj.k3284.long http://www.ncbi.nlm.nih.gov/pubmed/30097460?tool=bestpractice.com [179]Rochwerg B, Oczkowski SJ, Siemieniuk RAC, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018 Sep;46(9):1411-20. http://www.ncbi.nlm.nih.gov/pubmed/29979221?tool=bestpractice.com This effect was seen in sepsis, with and without shock, although the greatest benefit from corticosteroids was among those with septic shock. The clinical practice guideline concluded that both corticosteroid and non-corticosteroid management approaches are appropriate and suggested that the patient's values and preferences may guide the decision.[178]Lamontagne F, Rochwerg B, Lytvyn L, et al. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018 Aug 10;362:k3284. https://www.bmj.com/content/362/bmj.k3284.long http://www.ncbi.nlm.nih.gov/pubmed/30097460?tool=bestpractice.com Patients who prioritize living over quality of life would likely choose to have corticosteroid treatment, whereas those who place more value on avoiding functional deterioration and maximizing quality of life than on avoiding death may be more likely to choose not to have corticosteroids.[178]Lamontagne F, Rochwerg B, Lytvyn L, et al. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018 Aug 10;362:k3284. https://www.bmj.com/content/362/bmj.k3284.long http://www.ncbi.nlm.nih.gov/pubmed/30097460?tool=bestpractice.com [179]Rochwerg B, Oczkowski SJ, Siemieniuk RAC, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018 Sep;46(9):1411-20. http://www.ncbi.nlm.nih.gov/pubmed/29979221?tool=bestpractice.com The 2021 Surviving Sepsis Campaign guideline includes a weak recommendation to administer intravenous corticosteroids in adults with septic shock and an ongoing requirement for vasopressor therapy.[3]Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-143. https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/34605781?tool=bestpractice.com
Primary options
hydrocortisone sodium succinate: 50 mg intravenously every 6 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
hydrocortisone sodium succinate: 50 mg intravenously every 6 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
hydrocortisone sodium succinate
Plus – broad-spectrum respiratory pathogen cover to include atypicals
broad-spectrum respiratory pathogen cover to include atypicals
Treatment recommended for ALL patients in selected patient group
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guidelines recommend that combination therapy for patients admitted to the hospital should include a beta-lactam such as cefotaxime, ceftriaxone, ceftaroline, or ampicillin/sulbactam, with a macrolide such as azithromycin.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com Although ATS/IDSA recommend clarithromycin in these patients, it is only available as an oral formulation in the US so is unlikely to be useful in this setting. A beta-lactam and a fluoroquinolone (e.g., moxifloxacin, levofloxacin) combination may be given to patients with severe community-acquired pneumonia. There is stronger evidence for the beta-lactam plus macrolide combination.
Fluoroquinolone antibiotics should be avoided when effective and appropriate alternatives are available and can be given promptly. Systemic fluoroquinolone antibiotics may cause serious, disabling, and potentially long-lasting or irreversible adverse events. This includes, but is not limited to: tendinopathy/tendon rupture; peripheral neuropathy; arthropathy/arthralgia; aortic aneurysm and dissection; heart valve regurgitation; dysglycemia; and central nervous system effects including seizures, depression, psychosis, and suicidal thoughts and behavior.[142]Rusu A, Munteanu AC, Arbănași EM, et al. Overview of side-effects of antibacterial fluoroquinolones: new drugs versus old drugs, a step forward in the safety profile? Pharmaceutics. 2023 Mar 1;15(3):804. https://www.doi.org/10.3390/pharmaceutics15030804 http://www.ncbi.nlm.nih.gov/pubmed/36986665?tool=bestpractice.com Prescribing restrictions apply to the use of fluoroquinolones, and these restrictions may vary between countries. In general, fluoroquinolones should be restricted for use in serious, life-threatening bacterial infections only. Some regulatory agencies may also recommend that they must only be used in situations where other antibiotics that are commonly recommended for the infection are inappropriate (e.g., resistance, contraindications, treatment failure, unavailability). Consult your local guidelines and drug information source for more information on suitability, contraindications, and precautions.
Antibiotic therapy should be given for a minimum of 5 days.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
Primary options
ampicillin/sulbactam: 1.5 to 3 g intravenously every 6 hours
More ampicillin/sulbactamDose consists of 1 g of ampicillin plus 0.5 g sulbactam (1.5 g dose) or 2 g of ampicillin plus 1 g sulbactam (3 g dose).
or
cefotaxime: 1-2 g intravenously every 8 hours
or
ceftriaxone: 1-2 g intravenously every 24 hours
or
ceftaroline fosamil: 600 mg intravenously every 12 hours
-- AND --
azithromycin: 500 mg intravenously every 24 hours
Secondary options
ampicillin/sulbactam: 1.5 to 3 g intravenously every 6 hours
More ampicillin/sulbactamDose consists of 1 g of ampicillin plus 0.5 g sulbactam (1.5 g dose) or 2 g of ampicillin plus 1 g sulbactam (3 g dose).
or
cefotaxime: 1-2 g intravenously every 8 hours
or
ceftriaxone: 1-2 g intravenously every 24 hours
or
ceftaroline fosamil: 600 mg intravenously every 12 hours
-- AND --
levofloxacin: 750 mg intravenously every 24 hours
or
moxifloxacin: 400 mg intravenously every 24 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
ampicillin/sulbactam: 1.5 to 3 g intravenously every 6 hours
More ampicillin/sulbactamDose consists of 1 g of ampicillin plus 0.5 g sulbactam (1.5 g dose) or 2 g of ampicillin plus 1 g sulbactam (3 g dose).
or
cefotaxime: 1-2 g intravenously every 8 hours
or
ceftriaxone: 1-2 g intravenously every 24 hours
or
ceftaroline fosamil: 600 mg intravenously every 12 hours
-- AND --
azithromycin: 500 mg intravenously every 24 hours
Secondary options
ampicillin/sulbactam: 1.5 to 3 g intravenously every 6 hours
More ampicillin/sulbactamDose consists of 1 g of ampicillin plus 0.5 g sulbactam (1.5 g dose) or 2 g of ampicillin plus 1 g sulbactam (3 g dose).
or
cefotaxime: 1-2 g intravenously every 8 hours
or
ceftriaxone: 1-2 g intravenously every 24 hours
or
ceftaroline fosamil: 600 mg intravenously every 12 hours
-- AND --
levofloxacin: 750 mg intravenously every 24 hours
or
moxifloxacin: 400 mg intravenously every 24 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
ampicillin/sulbactam
or
cefotaxime
or
ceftriaxone
or
ceftaroline fosamil
-- AND --
azithromycin
Secondary options
ampicillin/sulbactam
or
cefotaxime
or
ceftriaxone
or
ceftaroline fosamil
-- AND --
levofloxacin
or
moxifloxacin
Consider – Pseudomonas aeruginosa antibiotic cover (if needed)
Pseudomonas aeruginosa antibiotic cover (if needed)
Treatment recommended for SOME patients in selected patient group
Additional empiric antibiotic cover is required in patients with risk factors for Pseudomonas aeruginosa if locally validated risk factors are present. These include hospitalization, prior respiratory isolation of Pseudomonas, or systemic antibiotic use within the previous 90 days.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
Patients who are at risk of Pseudomonas infection and who have severe pneumonia, or who have prior respiratory isolation of P aeruginosa, should be treated with piperacillin/tazobactam, cefepime, ceftazidime, imipenem/cilastatin, meropenem, or aztreonam addition to an empiric beta-lactam combination regimen.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
American Thoracic Society/Infectious Diseases Society of America guidelines advise that most multidrug-resistant organisms that cause community-acquired pneumonia are effectively covered by antibiotic regimens for Pseudomonas.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
Primary options
piperacillin/tazobactam: 4.5 g intravenously every 6 hours
More piperacillin/tazobactamDose consists of 4 g piperacillin plus 0.5 g tazobactam.
OR
cefepime: 2 g intravenously every 8 hours
OR
ceftazidime sodium: 2 g intravenously every 8 hours
OR
imipenem/cilastatin: 500 mg intravenously every 6 hours
More imipenem/cilastatinDose refers to imipenem component.
OR
meropenem: 1 g intravenously every 8 hours
OR
aztreonam: 2 g intravenously every 8 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
piperacillin/tazobactam: 4.5 g intravenously every 6 hours
More piperacillin/tazobactamDose consists of 4 g piperacillin plus 0.5 g tazobactam.
OR
cefepime: 2 g intravenously every 8 hours
OR
ceftazidime sodium: 2 g intravenously every 8 hours
OR
imipenem/cilastatin: 500 mg intravenously every 6 hours
More imipenem/cilastatinDose refers to imipenem component.
OR
meropenem: 1 g intravenously every 8 hours
OR
aztreonam: 2 g intravenously every 8 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
piperacillin/tazobactam
OR
cefepime
OR
ceftazidime sodium
OR
imipenem/cilastatin
OR
meropenem
OR
aztreonam
MRSA antibiotic cover (if needed)
Treatment recommended for SOME patients in selected patient group
Additional empiric antibiotic cover is required in patients with risk factors for MRSA if locally validated risk factors are present. These include hospitalization, prior respiratory isolation of MRSA, or systemic antibiotic use within the previous 90 days.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
In patients at risk of MRSA who have severe pneumonia, or who have prior respiratory isolation of MRSA, vancomycin or linezolid should be added to an empiric beta-lactam combination.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
Primary options
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
OR
linezolid: 600 mg intravenously every 12 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
OR
linezolid: 600 mg intravenously every 12 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
vancomycin
OR
linezolid
Consider – Enterobacteriaceae antibiotic cover (if needed)
Enterobacteriaceae antibiotic cover (if needed)
Treatment recommended for SOME patients in selected patient group
Additional empiric antibiotic cover is required in patients with risk factors for extended-spectrum beta-lactamase-producing Enterobacteriaceae. Consult an infectious disease specialist for guidance on an appropriate antibiotic regimen.[143]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
Plus – broad-spectrum cover for predominant gram-negative coliforms and Pseudomonas
broad-spectrum cover for predominant gram-negative coliforms and Pseudomonas
Treatment recommended for ALL patients in selected patient group
If urinary stasis is present, drainage is of immediate importance.
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
A fluoroquinolone (e.g., ciprofloxacin) may be used in patients with penicillin allergy if local sensitivity patterns are taken into account. Fluoroquinolone antibiotics should be avoided when effective and appropriate alternatives are available and can be given promptly. Systemic fluoroquinolone antibiotics may cause serious, disabling, and potentially long-lasting or irreversible adverse events. This includes, but is not limited to: tendinopathy/tendon rupture; peripheral neuropathy; arthropathy/arthralgia; aortic aneurysm and dissection; heart valve regurgitation; dysglycemia; and central nervous system effects including seizures, depression, psychosis, and suicidal thoughts and behavior.[142]Rusu A, Munteanu AC, Arbănași EM, et al. Overview of side-effects of antibacterial fluoroquinolones: new drugs versus old drugs, a step forward in the safety profile? Pharmaceutics. 2023 Mar 1;15(3):804. https://www.doi.org/10.3390/pharmaceutics15030804 http://www.ncbi.nlm.nih.gov/pubmed/36986665?tool=bestpractice.com Prescribing restrictions apply to the use of fluoroquinolones, and these restrictions may vary between countries. In general, fluoroquinolones should be restricted for use in serious, life-threatening bacterial infections only. Some regulatory agencies may also recommend that they must only be used in situations where other antibiotics that are commonly recommended for the infection are inappropriate (e.g., resistance, contraindications, treatment failure, unavailability). Consult your local guidelines and drug information source for more information on suitability, contraindications, and precautions.
Primary options
No penicillin allergy
ampicillin: 1-2 g intravenously every 6 hours
or
ceftriaxone: 1 g intravenously every 24 hours
or
cefotaxime: 1-2 g intravenously every 8 hours
-- AND --
gentamicin: 5 mg/kg intravenously every 24 hours
More gentamicinAdjust dose according to serum gentamicin level.
Secondary options
Penicillin allergy
ciprofloxacin: 400 mg intravenously every 12 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
No penicillin allergy
ampicillin: 1-2 g intravenously every 6 hours
or
ceftriaxone: 1 g intravenously every 24 hours
or
cefotaxime: 1-2 g intravenously every 8 hours
-- AND --
gentamicin: 5 mg/kg intravenously every 24 hours
More gentamicinAdjust dose according to serum gentamicin level.
Secondary options
Penicillin allergy
ciprofloxacin: 400 mg intravenously every 12 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
No penicillin allergy
ampicillin
or
ceftriaxone
or
cefotaxime
-- AND --
gentamicin
Secondary options
Penicillin allergy
ciprofloxacin
Plus – broad-spectrum cover for gram-positive and gram-negative organisms including anaerobes
broad-spectrum cover for gram-positive and gram-negative organisms including anaerobes
Treatment recommended for ALL patients in selected patient group
Control and drainage of an intra-abdominal source of infection is of immediate importance.
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
A fluoroquinolone (e.g., ciprofloxacin, levofloxacin) may be used in patients with penicillin allergy if local sensitivity patterns are taken into account. Fluoroquinolone antibiotics should be avoided when effective and appropriate alternatives are available and can be given promptly. Systemic fluoroquinolone antibiotics may cause serious, disabling, and potentially long-lasting or irreversible adverse events. This includes, but is not limited to: tendinopathy/tendon rupture; peripheral neuropathy; arthropathy/arthralgia; aortic aneurysm and dissection; heart valve regurgitation; dysglycemia; and central nervous system effects including seizures, depression, psychosis, and suicidal thoughts and behavior.[142]Rusu A, Munteanu AC, Arbănași EM, et al. Overview of side-effects of antibacterial fluoroquinolones: new drugs versus old drugs, a step forward in the safety profile? Pharmaceutics. 2023 Mar 1;15(3):804. https://www.doi.org/10.3390/pharmaceutics15030804 http://www.ncbi.nlm.nih.gov/pubmed/36986665?tool=bestpractice.com Prescribing restrictions apply to the use of fluoroquinolones, and these restrictions may vary between countries. In general, fluoroquinolones should be restricted for use in serious, life-threatening bacterial infections only. Some regulatory agencies may also recommend that they must only be used in situations where other antibiotics, that are commonly recommended for the infection, are inappropriate (e.g., resistance, contraindications, treatment failure, unavailability). Consult your local guidelines and drug information source for more information on suitability, contraindications, and precautions.
Primary options
No penicillin allergy
ceftazidime sodium: 2 g intravenously every 8 hours
or
cefepime: 2 g intravenously every 8-12 hours
-- AND --
metronidazole: 500 mg intravenously every 8 hours
OR
No penicillin allergy
piperacillin/tazobactam: 3.375 to 4.5 g intravenously every 6 hours
More piperacillin/tazobactamDose consists of 3 g piperacillin plus 0.375 g tazobactam (3.375 g dose) or 4 g piperacillin plus 0.5 g tazobactam (4.5 g dose).
Secondary options
Penicillin allergy
ciprofloxacin: 400 mg intravenously every 12 hours
or
levofloxacin: 750 mg intravenously every 24 hours
-- AND --
metronidazole: 500 mg intravenously every 8 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
No penicillin allergy
ceftazidime sodium: 2 g intravenously every 8 hours
or
cefepime: 2 g intravenously every 8-12 hours
-- AND --
metronidazole: 500 mg intravenously every 8 hours
OR
No penicillin allergy
piperacillin/tazobactam: 3.375 to 4.5 g intravenously every 6 hours
More piperacillin/tazobactamDose consists of 3 g piperacillin plus 0.375 g tazobactam (3.375 g dose) or 4 g piperacillin plus 0.5 g tazobactam (4.5 g dose).
Secondary options
Penicillin allergy
ciprofloxacin: 400 mg intravenously every 12 hours
or
levofloxacin: 750 mg intravenously every 24 hours
-- AND --
metronidazole: 500 mg intravenously every 8 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
No penicillin allergy
ceftazidime sodium
or
cefepime
-- AND --
metronidazole
OR
No penicillin allergy
piperacillin/tazobactam
Secondary options
Penicillin allergy
ciprofloxacin
or
levofloxacin
-- AND --
metronidazole
azole or echinocandin antifungal
Treatment recommended for SOME patients in selected patient group
Patients with recurrent perforation of the large intestine are at increased risk of invasive fungemia. An azole, such as fluconazole, or an echinocandin, such as micafungin, should be added to the antibacterial cover. Non-albicans species of Candida are increasingly resistant to azoles.[47]Solomkin JS, Mazuski J. Intra-abdominal sepsis: newer interventional and antimicrobial therapies. Infect Dis Clin North Am. 2009 Sep;23(3):593-608. http://www.ncbi.nlm.nih.gov/pubmed/19665085?tool=bestpractice.com [48]Montravers P, Dupont H, Gauzit R, et al. Candida as a risk factor for mortality in peritonitis. Crit Care Med. 2006 Mar;34(3):646-52. http://www.ncbi.nlm.nih.gov/pubmed/16505648?tool=bestpractice.com [146]Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Feb 15;62(4):e1-50. https://academic.oup.com/cid/article/62/4/e1/2462830 http://www.ncbi.nlm.nih.gov/pubmed/26679628?tool=bestpractice.com
Primary options
fluconazole: consult specialist for guidance on dose
OR
micafungin: consult specialist for guidance on dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
fluconazole: consult specialist for guidance on dose
OR
micafungin: consult specialist for guidance on dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
fluconazole
OR
micafungin
Plus – broad-spectrum gram-positive and gram-negative cover including anaerobes
broad-spectrum gram-positive and gram-negative cover including anaerobes
Treatment recommended for ALL patients in selected patient group
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only. Metronidazole should be considered as empiric therapy if anaerobic infection is suspected.
Primary options
No penicillin allergy
nafcillin: 1-2 g intravenously every 4-6 hours
and
metronidazole: 500 mg intravenously every 8 hours
Secondary options
Penicillin allergy
clindamycin: 600-900 mg intravenously every 8 hours
OR
Penicillin allergy
tigecycline: 100 mg intravenously initially, followed by 50 mg every 12 hours
and
metronidazole: 500 mg intravenously every 8 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
No penicillin allergy
nafcillin: 1-2 g intravenously every 4-6 hours
and
metronidazole: 500 mg intravenously every 8 hours
Secondary options
Penicillin allergy
clindamycin: 600-900 mg intravenously every 8 hours
OR
Penicillin allergy
tigecycline: 100 mg intravenously initially, followed by 50 mg every 12 hours
and
metronidazole: 500 mg intravenously every 8 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
No penicillin allergy
nafcillin
and
metronidazole
Secondary options
Penicillin allergy
clindamycin
OR
Penicillin allergy
tigecycline
and
metronidazole
broad-spectrum cover to include MRSA
Treatment recommended for ALL patients in selected patient group
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
In cases of suspected MRSA-associated soft-tissue or joint infections, vancomycin or linezolid is typically added to the antibiotic therapy.
Primary options
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
OR
linezolid: 600 mg intravenously every 12 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
OR
linezolid: 600 mg intravenously every 12 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
vancomycin
OR
linezolid
Plus – broad-spectrum cover to include group A Streptococci and gram-negative organisms
broad-spectrum cover to include group A Streptococci and gram-negative organisms
Treatment recommended for ALL patients in selected patient group
If necrotizing fasciitis is suspected, surgical control and debridement is of immediate importance.
Once culture and sensitivity results are known, antibiotics should be adjusted if required; 90% of cases are polymicrobial.
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
Primary options
No penicillin allergy
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
and
piperacillin/tazobactam: 3.375 g intravenously every 6 hours, or 4.5 g intravenously every 6-8 hours
More piperacillin/tazobactamDose consists of 3 g piperacillin plus 0.375 g tazobactam (3.375 g dose) or 4 g piperacillin plus 0.5 g tazobactam (4.5 g dose).
and
clindamycin: 600-900 mg intravenously every 8 hours
Secondary options
No penicillin allergy
nafcillin: 1-2 g intravenously every 6 hours
and
clindamycin: 600-900 mg intravenously every 8 hours
OR
Penicillin allergy
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
-- AND --
meropenem: 1 g intravenously every 8 hours
or
ertapenem: 1 g intravenously every 24 hours
-- AND --
clindamycin: 600-900 mg intravenously every 8 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
No penicillin allergy
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
and
piperacillin/tazobactam: 3.375 g intravenously every 6 hours, or 4.5 g intravenously every 6-8 hours
More piperacillin/tazobactamDose consists of 3 g piperacillin plus 0.375 g tazobactam (3.375 g dose) or 4 g piperacillin plus 0.5 g tazobactam (4.5 g dose).
and
clindamycin: 600-900 mg intravenously every 8 hours
Secondary options
No penicillin allergy
nafcillin: 1-2 g intravenously every 6 hours
and
clindamycin: 600-900 mg intravenously every 8 hours
OR
Penicillin allergy
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
-- AND --
meropenem: 1 g intravenously every 8 hours
or
ertapenem: 1 g intravenously every 24 hours
-- AND --
clindamycin: 600-900 mg intravenously every 8 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
No penicillin allergy
vancomycin
and
piperacillin/tazobactam
and
clindamycin
Secondary options
No penicillin allergy
nafcillin
and
clindamycin
OR
Penicillin allergy
vancomycin
-- AND --
meropenem
or
ertapenem
-- AND --
clindamycin
broad-spectrum cover to include Neisseria meningitidis, Streptococcus pneumoniae, and other common gram-positive causative agents
Treatment recommended for ALL patients in selected patient group
Suspected meningitis or meningococcal septicemia should be treated immediately using a third-generation cephalosporin (e.g., ceftriaxone, cefotaxime) in combination with vancomycin.[147]Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004 Nov 1;39(9):1267-84. https://academic.oup.com/cid/article/39/9/1267/402080?login=false http://www.ncbi.nlm.nih.gov/pubmed/15494903?tool=bestpractice.com [148]van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016 May;22 (suppl 3):S37-62. https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(16)00020-3/fulltext http://www.ncbi.nlm.nih.gov/pubmed/27062097?tool=bestpractice.com For patients with penicillin allergy, vancomycin with chloramphenicol is a suitable alternative. Some suggest the addition of rifampin to either regimen to aid penetration.
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
Primary options
No penicillin allergy
ceftriaxone: 2 g intravenously every 12 hours
or
cefotaxime: 2 g intravenously every 4-6 hours
-- AND --
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
Secondary options
Penicillin allergy
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
and
chloramphenicol: 1 g intravenously every 6 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
No penicillin allergy
ceftriaxone: 2 g intravenously every 12 hours
or
cefotaxime: 2 g intravenously every 4-6 hours
-- AND --
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
Secondary options
Penicillin allergy
vancomycin: 15-20 mg/kg intravenously every 8-12 hours
More vancomycinAdjust dose according to serum vancomycin level.
and
chloramphenicol: 1 g intravenously every 6 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
No penicillin allergy
ceftriaxone
or
cefotaxime
-- AND --
vancomycin
Secondary options
Penicillin allergy
vancomycin
and
chloramphenicol
rifampin
Treatment recommended for SOME patients in selected patient group
Some experts suggest the addition of rifampin to the standard antibiotic regimen to aid penetration.
Primary options
rifampin: 600 mg intravenously every 24 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
rifampin: 600 mg intravenously every 24 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
rifampin
antibiotic cover for Listeria
Treatment recommended for SOME patients in selected patient group
For patients over 50 years and those with history of alcohol use disorder or other debilitating illness, or at increased risk of infection with Listeria (e.g., pregnant women, immunosuppression), cephalosporins alone provide inadequate cover. Ampicillin should be added to the regimen, provided the patient is not allergic to penicillin. For those allergic to penicillin, erythromycin or trimethoprim/sulfamethoxazole can be substituted.
Primary options
No penicillin allergy
ampicillin: 2 g intravenously every 6 hours
Secondary options
Penicillin allergy
erythromycin lactobionate: 1 g intravenously every 6 hours
OR
Penicillin allergy
sulfamethoxazole/trimethoprim: 20 mg/kg/day intravenously given in divided doses every 6 hours
More sulfamethoxazole/trimethoprimDose refers to trimethoprim component.
These drug options and doses relate to a patient with no comorbidities.
Primary options
No penicillin allergy
ampicillin: 2 g intravenously every 6 hours
Secondary options
Penicillin allergy
erythromycin lactobionate: 1 g intravenously every 6 hours
OR
Penicillin allergy
sulfamethoxazole/trimethoprim: 20 mg/kg/day intravenously given in divided doses every 6 hours
More sulfamethoxazole/trimethoprimDose refers to trimethoprim component.
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
No penicillin allergy
ampicillin
Secondary options
Penicillin allergy
erythromycin lactobionate
OR
Penicillin allergy
sulfamethoxazole/trimethoprim
antiviral cover for herpes simplex virus (HSV)
Treatment recommended for SOME patients in selected patient group
For patients in whom HSV encephalitis is suspected (symptoms of confusion, seizure, or with cerebrospinal fluid findings consistent with viral meningitis), empiric coverage with acyclovir is recommended.
Primary options
acyclovir: 10 mg/kg intravenously every 8 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
acyclovir: 10 mg/kg intravenously every 8 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
acyclovir
Plus – broad-spectrum gram-positive and gram-negative cover
broad-spectrum gram-positive and gram-negative cover
Treatment recommended for ALL patients in selected patient group
Intensive efforts, including imaging, should be undertaken to attempt to evaluate the source of infection. Urgent broad-spectrum coverage to include all common pathogens should be administered.
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only.
Primary options
imipenem/cilastatin: 1 g intravenously every 8 hours
More imipenem/cilastatinDose refers to imipenem component.
OR
meropenem: 1 g intravenously every 8 hours
OR
These drug options and doses relate to a patient with no comorbidities.
Primary options
imipenem/cilastatin: 1 g intravenously every 8 hours
More imipenem/cilastatinDose refers to imipenem component.
OR
meropenem: 1 g intravenously every 8 hours
OR
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
imipenem/cilastatin
OR
meropenem
OR
piperacillin/tazobactam
and
gentamicin
broad-spectrum cover to include MRSA
Treatment recommended for ALL patients in selected patient group
Intensive efforts, including imaging, should be undertaken to attempt to evaluate the source of infection. Urgent broad-spectrum coverage to include all common pathogens should be administered.
When choosing empiric therapy, the suspected source of infection or causative organism, local resistance patterns, and the patient's immune status need to be considered.[60]Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000 Apr;16(2):179-92. http://www.ncbi.nlm.nih.gov/pubmed/10768078?tool=bestpractice.com Antibiotics listed are suggested as guidance only. Vancomycin is used if MRSA is suspected.
These drug options and doses relate to a patient with no comorbidities.
Primary options
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
vancomycin
-- AND --
imipenem/cilastatin
or
meropenem

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer