Approach
Diagnosis of this potentially fatal disease rests on a high index of suspicion in a patient with suggestive symptoms and an appropriate travel history. While epidemiological maps of malaria endemicity may be useful in determining risk for the purpose of pre-travel advice regarding prophylaxis, such an approach may be dangerous in the setting of managing a febrile patient returning from an area bordering a malaria area; conditions on the ground can change.
The differential diagnosis is broad and includes other travel-related infections. In resource-poor settings a presumptive diagnosis is often made on clinical grounds alone. However, as symptoms are nonspecific, clinical diagnosis is unreliable. Therefore, where diagnostic testing is available, confirmation of infection should always be sought as soon as possible.
Treatment depends on the infecting species, the parasite burden, and the clinical status of the patient; thus, history, examination, and laboratory tests should focus on determining these factors.
Most countries require reporting of malaria so that epidemiologic data can be collected. In the US, healthcare providers should report all cases of laboratory-confirmed malaria occurring in the US and its territories to their local or state health department. CDC: how to report a case of malaria Opens in new window
History
Most patients with Plasmodium falciparum infection present in the first month after exposure; almost all present within 3 months of exposure. Plasmodium vivax or Plasmodium ovale infections commonly present later than 3 months after exposure, and presentation may even be delayed for 1 year or more.[29]
Symptoms are nonspecific and common to many other infections. Fever, or history of fever, is universal. Characteristic paroxysms of chills and rigors followed by fever and sweats may be described. Patterns of fever are rarely diagnostic at presentation but may develop over time: fevers occurring at regular intervals of 48 hours may be associated with P vivax or P ovale infection, and at intervals of 72 hours with P malariae infection. Other very common symptoms include headache, weakness, myalgia, and arthralgia. Less common symptoms include anorexia, nausea, vomiting, diarrhea, and abdominal pain. History should also focus on whether risk factors for severe disease are present (e.g., low host immunity, pregnancy, children ages <5 years, immunocompromise, malnutrition, older age).
History of travel to an endemic area (especially visiting friends and relatives) is the key diagnostic factor in patients presenting with fever in nonendemic countries. However, travel history is frequently overlooked. History of travel to a malaria endemic area in the past 12 months should be elicited if a patient presents with nonspecific symptoms. If travel history is positive, the patient should be asked if antimalarial prophylaxis was taken. If taken, the specific drug should be determined and whether it was taken in the correct manner. As reporting is often unreliable, the use of prophylaxis does not exclude infection; however, it may influence selection of the treatment regimen if malaria is confirmed.
Physical examination
Clinical status of the patient should be assessed. There are very few physical signs to elicit that are specific for malaria. It is important to determine whether a complication of severe malaria is present (suggested by jaundice, confusion or altered level of consciousness, prostration, seizures, hypotension, respiratory distress, bleeding, or anuria/oliguria) or whether there is concurrent bacterial infection (suggested by presence of focal signs, such as crackles on lung auscultation suggesting super-imposed pneumonia). Signs often present include hepatosplenomegaly (although not common at time of initial presentation in returning travelers) and/or pallor (suggesting anemia). Tachypnea may indicate severe malaria with acidosis.
Coinfections (including bacterial, viral, fungal, and parasitic infections) are common in endemic countries, and depend on the geographic area. Vigilance is required for clinical features that are atypical of malaria, especially in areas where resources are constrained and there is limited diagnostic capacity.[72]
Laboratory investigations
Light microscopy
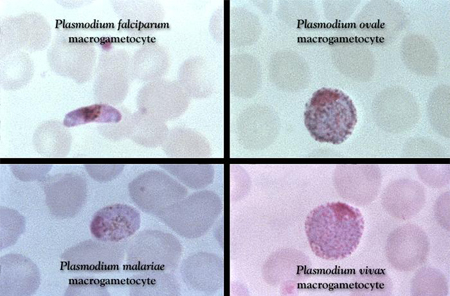
The detection of malaria parasites in the blood is essential. In skilled hands, the most sensitive and specific test readily available is light microscopy of Giemsa-stained blood smears, and this is the diagnostic test of choice. Wright or Field stains are alternatives to Giemsa. When to use these alternatives is a laboratory decision and may vary locally. Thick films enable a relatively large amount of blood to be screened for parasites, but species determination may be difficult as red cells are lysed and parasite morphology may be distorted. Thin film preparations allow speciation, which will affect treatment decisions. The percentage of parasitemia can be assessed using a simple formula but may be extremely low, and false-negative thick smears may occur.[73] If there is a strong suspicion of malaria, the test should be repeated on three separate occasions over 48 hours.[29][Figure caption and citation for the preceding image starts]: Giemsa-stained slide revealing Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, and Plasmodium vivax gametocytesCDC Image Library; used with permission [Citation ends].
Rapid diagnostic tests (RDTs)
RDTs are immunochromatographic tests that detect the presence of parasite antigens on a finger prick sample. Different types detect either P falciparum-specific histidine-rich protein-2 (HRP-2) or species-specific parasite lactate dehydrogenase (pLDH).[74] A number of tests have been evaluated in returned travelers, with most generating sensitivity and specificity values for P falciparum of over 85% to 90%.[74] Problems have included poor detection of species other than P falciparum and false-negative results at lower parasite burden.[74][75][76] However, some studies have shown that performance of RDTs can be equivalent to expert microscopy.[77][78]
The main advantage of RDTs is that they provide a means for rapid diagnosis. This may be particularly useful in health resource-limited areas where microscopy is not available or reliable. Their incorporation into treatment algorithms may substantially reduce prescribing of antimalarials when compared with clinical diagnosis alone.[79][80] [
 ]
[
]
[  ]
]
The disadvantages of HRP-2 RDTs include the inability to distinguish between active infection and recently treated infection. In addition, even with a positive HRP-2 RDTor pLDH RDT, a blood film is still necessary for confirmation of species (nonfalciparum) and for a parasite count to help guide treatment (falciparum and knowlesi). pLDH tests identify active infection. Another disadvantage is the emergence of pfhrp2/pfhrp3 gene deletions leading to false-negative test results in some areas (e.g., Horn of Africa).[81] False-negative test results may also occur in patients with very high parasite densities (the prozone effect), and this seems to be more common with HRP-2-based tests.[82]
The choice between RDTs and microscopy depends on local circumstances, including the skills available, patient caseload, and epidemiology of malaria.[49] A systematic review found that pLDH tests had a higher specificity, whereas the HRP-2 tests had better sensitivity and slightly greater accuracy, when compared with each other.[83] A combination of both tests, if possible, may be more reliable. Ultra-sensitive HRP-2 tests have been developed.[84]
Polymerase chain reaction (PCR)
PCR is usually only available in reference laboratories. It may be used to confirm the diagnosis of malaria in cases where microscopy is negative but there is a high clinical suspicion of disease, or to determine the species when it is not possible to distinguish on light microscopy, provided it can be done in a timely manner and does not delay diagnosis.[85][86]
In high-transmission settings, a substantial proportion of the population may have submicroscopic parasitemia detectable by PCR.[87] Asymptomatic parasite carriers can act as a reservoir for malaria transmission.
Loop-mediated isothermal amplification (LAMP)
LAMP is molecular assay that is more sensitive than microscopy or antigen-detecting RDTs, so is more reliable in detecting low parasitemia. It provides faster results than microscopy or PCR, requires less training, is less costly compared with PCR, and has been evaluated in both high- and low-resource settings.[88][89] It is still considered an emerging test.
Other baseline tests
Baseline tests include a complete blood count, clotting profile, renal and liver function tests, blood glucose, and urinalysis. In cases of severe malaria, blood gas analysis is necessary to exclude metabolic or lactic acidosis. HIV testing is indicated, as malaria may be more severe in the presence of HIV infection. If concurrent bacterial infection is suspected, blood, sputum, and urine cultures should be requested. Lumbar puncture may be indicated to exclude meningitis. In addition, PCR of nasopharyngeal swabs may be considered to exclude influenza or coronavirus disease 2019 (COVID-19) infection, which can present in an identical manner.
Imaging
A chest x-ray helps exclude other diagnoses (e.g., pneumonia, pulmonary tuberculosis) when investigating patients returning from abroad with a fever. In addition, a chest x-ray is indicated if there is suspicion of severe malaria, particularly with respiratory symptoms or signs, or if consciousness level is reduced (to exclude aspiration).
Computed tomography of the head is an important investigation to seek focal lesions or hemorrhage if there are focal neurologic signs, impaired level of consciousness, or seizures.
How to perform a diagnostic lumbar puncture in adults. Includes a discussion of patient positioning, choice of needle, and measurement of opening and closing pressure.
Use of this content is subject to our disclaimer
