Diagnosis of Zika virus infection is based on clinical suspicion along with molecular testing and, in some circumstances, serologic testing. Although the majority of infected people are asymptomatic, physicians should have a high index of suspicion for patients who present with fever, a maculopapular (sometimes morbilliform) rash, arthralgia/myalgia, and conjunctivitis in the correct epidemiologic context (i.e., residence in/travel from an area where there is a current outbreak or the potential for transmission). The diagnostic approach is different in pregnant and nonpregnant individuals.
The clinical spectrum of disease overlaps with that caused by other arbovirus infections. As a consequence, the differential diagnosis is broad and includes dengue and chikungunya infection. It is important to differentiate between Zika, dengue, and chikungunya virus infection as the three diseases can produce similar symptoms, particularly during the acute phase.[137]World Health Organization; Pan American Health Organization. Tool for the diagnosis and care of patients with suspected arboviral diseases. Mar 2017 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/33895
Coinfection with chikungunya and/or dengue is possible.[138]Lobkowicz L, Ramond A, Sanchez Clemente N, et al. The frequency and clinical presentation of Zika virus coinfections: a systematic review. BMJ Glob Health. 2020 May;5(5):e002350.
https://gh.bmj.com/content/5/5/e002350.long
http://www.ncbi.nlm.nih.gov/pubmed/32381652?tool=bestpractice.com
The World Health Organization (WHO) has produced a tool to help physicians differentiate between these three diseases.[137]World Health Organization; Pan American Health Organization. Tool for the diagnosis and care of patients with suspected arboviral diseases. Mar 2017 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/33895
Other conditions in the differential diagnosis, such as malaria or leptospirosis, may require specific and urgent treatment.
Congenital Zika syndrome is a recognized pattern of congenital anomalies in infants (i.e., microcephaly, intracranial calcifications or other brain anomalies, or eye anomalies, among others) associated with Zika virus infection during pregnancy.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
[3]França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016 Aug 27;388(10047):891-7.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)30902-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27372398?tool=bestpractice.com
[4]Melo AS, Aguiar RS, Amorim MM, et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol. 2016 Dec 1;73(12):1407-16.
http://jamanetwork.com/journals/jamaneurology/fullarticle/2557231
http://www.ncbi.nlm.nih.gov/pubmed/27695855?tool=bestpractice.com
[5]Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017 Mar 1;171(3):288-95.
https://jamanetwork.com/journals/jamapediatrics/fullarticle/2579543
http://www.ncbi.nlm.nih.gov/pubmed/27812690?tool=bestpractice.com
[6]Lucey D, Cummins H, Sholts S. Congenital Zika syndrome in 2017. JAMA. 2017 Apr 4;317(13):1368-9.
http://www.ncbi.nlm.nih.gov/pubmed/28384812?tool=bestpractice.com
According to the WHO, there is strong scientific consensus that Zika virus is a cause of microcephaly and these other congenital abnormalities.[7]Costello A, Dua T, Duran P, et al. Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ. 2016 Jun 1;94(6):406-406A.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4890216
http://www.ncbi.nlm.nih.gov/pubmed/27274588?tool=bestpractice.com
The Centers for Disease Control and Prevention (CDC) has also concluded that there is a causal relationship between prenatal Zika virus infections and microcephaly/other brain abnormalities.[8]Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects - reviewing the evidence for causality. N Engl J Med. 2016 May 19;374(20):1981-7.
http://www.nejm.org/doi/full/10.1056/NEJMsr1604338
http://www.ncbi.nlm.nih.gov/pubmed/27074377?tool=bestpractice.com
Evidence to support a causal relationship has expanded in recent years; however, the total number of cases investigated in the published cohort or case-control studies remains small.[9]Counotte MJ, Meili KW, Taghavi K, et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: a living systematic review. F1000Res. 2019 Aug 14;8:1433.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6852328
http://www.ncbi.nlm.nih.gov/pubmed/31754425?tool=bestpractice.com
Guillain-Barre syndrome and other neurologic disorders are strongly associated with and suspected to be caused by Zika virus infection but the link is unproven and studies are ongoing, including to elucidate a possible mechanism.[10]Dos Santos T, Rodriguez A, Almiron M, et al. Zika virus and the Guillain-Barré syndrome - case series from seven countries. N Engl J Med. 2016 Oct 20;375(16):1598-601.
http://www.nejm.org/doi/full/10.1056/NEJMc1609015?query=featured_zika
http://www.ncbi.nlm.nih.gov/pubmed/27579558?tool=bestpractice.com
[11]Parra B, Lizarazo J, Jiménez-Arango JA, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016 Oct 20;375(16):1513-23.
http://www.nejm.org/doi/full/10.1056/NEJMoa1605564#t=article
http://www.ncbi.nlm.nih.gov/pubmed/27705091?tool=bestpractice.com
[12]Frontera JA, da Silva IR. Zika getting on your nerves? The association with the Guillain-Barré syndrome. N Engl J Med. 2016 Oct 20;375(16):1581-2.
http://www.nejm.org/doi/full/10.1056/NEJMe1611840
http://www.ncbi.nlm.nih.gov/pubmed/27705077?tool=bestpractice.com
[13]Leis AA, Stokic DS. Zika virus and Guillain-Barre syndrome: is there sufficient evidence for causality? Front Neurol. 2016 Sep 30;7:170.
http://journal.frontiersin.org/article/10.3389/fneur.2016.00170/full
http://www.ncbi.nlm.nih.gov/pubmed/27746763?tool=bestpractice.com
Evidence to support a causal relationship has expanded in recent years; however, the body of evidence is still smaller than that for congenital abnormalities.[9]Counotte MJ, Meili KW, Taghavi K, et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: a living systematic review. F1000Res. 2019 Aug 14;8:1433.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6852328
http://www.ncbi.nlm.nih.gov/pubmed/31754425?tool=bestpractice.com
Infection prevention and control
Standard precautions (e.g., hand hygiene, use of personal protective equipment, respiratory hygiene and cough etiquette, safe injection practices, safe handling of potentially contaminated equipment or surfaces) are recommended for the protection of healthcare professionals and patients in healthcare settings and labor and delivery settings. These precautions are recommended regardless of whether the infection is suspected or confirmed.[92]Olson CK, Iwamoto M, Perkins KM, et al. Preventing transmission of Zika virus in labor and delivery settings through implementation of standard precautions - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016 Mar 25;65(11):290-2.
http://www.cdc.gov/mmwr/volumes/65/wr/mm6511e3.htm?s_cid=mm6511e3_w
http://www.ncbi.nlm.nih.gov/pubmed/27010422?tool=bestpractice.com
Transmission
Diagnosis should be suspected in patients who have resided in/traveled from an area where there is a current outbreak (or where the Aedes mosquito is present) in the 2 weeks prior to symptom onset. Nonvector transmission events (e.g., perinatal, in utero, sexual, and transfusion transmission) have also been reported.[58]Besnard M, Lastere S, Teissier A, et al. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014 Apr 3;19(13):20751.
http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20751
http://www.ncbi.nlm.nih.gov/pubmed/24721538?tool=bestpractice.com
[59]Ventura CV, Maia M, Ventura BV, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016 Feb;79(1):1-3.
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0004-27492016000100002&lng=en&nrm=iso&tlng=en
http://www.ncbi.nlm.nih.gov/pubmed/26840156?tool=bestpractice.com
[60]Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Possible association between Zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016 Jan 29;65(3):59-62.
http://dx.doi.org/10.15585/mmwr.mm6503e2
http://www.ncbi.nlm.nih.gov/pubmed/26820244?tool=bestpractice.com
[61]Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011 May;17(5):880-2.
http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/21529401
http://www.ncbi.nlm.nih.gov/pubmed/21529401?tool=bestpractice.com
[62]Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014 Apr 10;19(14):20761.
http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20761
http://www.ncbi.nlm.nih.gov/pubmed/24739982?tool=bestpractice.com
[63]Vasquez AM, Sapiano MR, Basavaraju SV, et al. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted Zika virus infection - Puerto Rico, 2016. MMWR Morb Mortal Wkly Rep. 2016 Apr 15;65(14):375-8.
http://www.cdc.gov/mmwr/volumes/65/wr/mm6514e1.htm?s_cid=mm6514e1_w
http://www.ncbi.nlm.nih.gov/pubmed/27078190?tool=bestpractice.com
[64]Barjas-Castro ML, Angerami RN, Cunha MS, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 2016 Jul;56(7):1684-8.
http://www.ncbi.nlm.nih.gov/pubmed/27329551?tool=bestpractice.com
[66]Motta IJ, Spencer BR, Cordeiro da Silva SG, et al. Evidence for transmission of Zika virus by platelet transfusion. N Engl J Med. 2016 Sep 15;375(11):1101-3.
http://www.nejm.org/doi/full/10.1056/NEJMc1607262
http://www.ncbi.nlm.nih.gov/pubmed/27532622?tool=bestpractice.com
[65]Petersen E, Wilson ME, Touch S, et al. Rapid spread of Zika virus in the Americas - implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic Games. Int J Infect Dis. 2016 Mar;44:11-5.
https://www.ijidonline.com/article/S1201-9712(16)00021-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26854199?tool=bestpractice.com
Sperm donation is a theoretical concern; however, there have been no reports as yet.
Clinical presentation
Approximately 80% of patients do not develop symptoms.[14]Pan American Health Organization; World Health Organization. Provisional remarks on Zika virus infection in pregnant women: document for health care professionals. Jan 2016 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/18600
[98]Centers for Disease Control and Prevention. Zika virus: clinical signs and symptoms of Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/clinical-signs/index.html
In those who do, the most common clinical findings include fever, an itchy maculopapular (sometimes morbilliform) rash, arthralgia, and nonpurulent conjunctivitis. The characteristic rash is one of the most distinctive symptoms.[14]Pan American Health Organization; World Health Organization. Provisional remarks on Zika virus infection in pregnant women: document for health care professionals. Jan 2016 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/18600
[46]Halani S, Tombindo PE, O'Reilly R, et al. Clinical manifestations and health outcomes associated with Zika virus infections in adults: a systematic review. PLoS Negl Trop Dis. 2021 Jul;15(7):e0009516.
https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0009516
http://www.ncbi.nlm.nih.gov/pubmed/34252102?tool=bestpractice.com
[98]Centers for Disease Control and Prevention. Zika virus: clinical signs and symptoms of Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/clinical-signs/index.html
[Figure caption and citation for the preceding image starts]: Characteristic maculopapular rash in a pregnant woman with Zika virus infectionFrom the personal collection of Dr Geraldo Furtado, MD, MSc (used with permission) [Citation ends].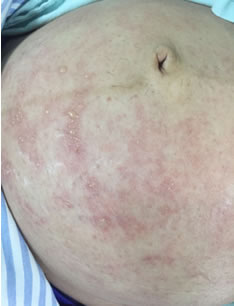
Other commonly reported symptoms include myalgia, malaise, and headache.[14]Pan American Health Organization; World Health Organization. Provisional remarks on Zika virus infection in pregnant women: document for health care professionals. Jan 2016 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/18600
[98]Centers for Disease Control and Prevention. Zika virus: clinical signs and symptoms of Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/clinical-signs/index.html
[129]World Health Organization. Zika virus fact sheet. Dec 2022 [internet publication].
https://www.who.int/en/news-room/fact-sheets/detail/zika-virus
Less common symptoms include vomiting/diarrhea, abdominal pain, anorexia, edema of the lower limbs, and retro-orbital pain.[14]Pan American Health Organization; World Health Organization. Provisional remarks on Zika virus infection in pregnant women: document for health care professionals. Jan 2016 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/18600
No differences in clinical presentation have been described between pregnant women and nonpregnant patients, or between adults and children. Most children have a rash, and more than half have a fever and rash.[139]Goodman AB, Dziuban EJ, Powell K, et al. Characteristics of children aged <18 Years with Zika virus disease acquired postnatally - US states, January 2015-July 2016. MMWR Morb Mortal Wkly Rep. 2016 Oct 7;65(39):1082-5.
https://www.cdc.gov/mmwr/volumes/65/wr/mm6539e2.htm?s_cid=mm6539e2_w
http://www.ncbi.nlm.nih.gov/pubmed/27711041?tool=bestpractice.com
The most common symptoms in children and adolescents were mild and included fever, rash, conjunctivitis, and arthralgia.[140]Ramond A, Lobkowicz L, Clemente NS, et al. Postnatal symptomatic Zika virus infections in children and adolescents: a systematic review. PLoS Negl Trop Dis. 2020 Oct;14(10):e0008612.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7556487
http://www.ncbi.nlm.nih.gov/pubmed/33006989?tool=bestpractice.com
Symptoms generally develop within 1 week of infection in 50% of patients, and within 2 weeks of infection in 99% of patients.[97]Krow-Lucal ER, Biggerstaff BJ, Staples JE. Estimated incubation period for Zika virus disease. Emerg Infect Dis. 2017 May;23(5):841-5.
https://wwwnc.cdc.gov/eid/article/23/5/16-1715_article
http://www.ncbi.nlm.nih.gov/pubmed/28277198?tool=bestpractice.com
Clinical illness is usually self-limited with mild symptoms lasting 2 to 7 days.[14]Pan American Health Organization; World Health Organization. Provisional remarks on Zika virus infection in pregnant women: document for health care professionals. Jan 2016 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/18600
[141]Lucey DR. Time for global action on Zika virus epidemic. BMJ. 2016 Feb 8;352:i781.
http://www.bmj.com/content/352/bmj.i781
http://www.ncbi.nlm.nih.gov/pubmed/26856896?tool=bestpractice.com
Severe disease requiring hospitalization is uncommon and has been estimated to be 11%, and the case fatality is low (median 0.02%).[14]Pan American Health Organization; World Health Organization. Provisional remarks on Zika virus infection in pregnant women: document for health care professionals. Jan 2016 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/18600
[46]Halani S, Tombindo PE, O'Reilly R, et al. Clinical manifestations and health outcomes associated with Zika virus infections in adults: a systematic review. PLoS Negl Trop Dis. 2021 Jul;15(7):e0009516.
https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0009516
http://www.ncbi.nlm.nih.gov/pubmed/34252102?tool=bestpractice.com
[142]Cardona-Ospina JA, Henao-SanMartin V, Acevedo-Mendoza WF, et al. Fatal Zika virus infection in the Americas: a systematic review. Int J Infect Dis. 2019 Nov;88:49-59.
https://www.ijidonline.com/article/S1201-9712(19)30361-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31499212?tool=bestpractice.com
Neurologic exam should be performed on all patients with suspected Guillain-Barre syndrome (GBS).
Neurologic complications are rare and have been reported in 0.3% of cases, with the most common complication being GBS.[46]Halani S, Tombindo PE, O'Reilly R, et al. Clinical manifestations and health outcomes associated with Zika virus infections in adults: a systematic review. PLoS Negl Trop Dis. 2021 Jul;15(7):e0009516.
https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0009516
http://www.ncbi.nlm.nih.gov/pubmed/34252102?tool=bestpractice.com
The clinical phenotype is generally a sensorimotor demyelinating GBS with a severe disease course. The most common features were limb weakness (97%), absent/diminished reflexes (96%), sensory symptoms (82%), and facial palsy (51%). Median time between infectious and neurologic symptoms was 5 to 12 days.[138]Lobkowicz L, Ramond A, Sanchez Clemente N, et al. The frequency and clinical presentation of Zika virus coinfections: a systematic review. BMJ Glob Health. 2020 May;5(5):e002350.
https://gh.bmj.com/content/5/5/e002350.long
http://www.ncbi.nlm.nih.gov/pubmed/32381652?tool=bestpractice.com
Key diagnostic factors include paresthesias (usually of the hands and feet), muscle weakness, pain (usually starts in the back and legs), and paralysis. Oropharyngeal, facial, and extraocular weakness may also occur. The WHO recommends using the Brighton criteria for the case definition of GBS.[143]World Health Organization. Assesssment and management of Guillain-Barré syndrome in the context of Zika virus: interim guidance update. Aug 2016 [internet publication].
https://www.who.int/publications/i/item/WHO-ZIKV-MOC-16.4-Rev.1
The Pan American Health Organization (PAHO) has also published a case definition for Zika-related GBS.[144]Pan American Health Organization. Case definitions. Apr 2016 [internet publication].
https://www.paho.org/en/documents/case-definitions-clinical-classification-and-disease-phases-dengue-chikungunya-and-zika
Physicians should be vigilant for early signs and symptoms of GBS as it may progress faster than usual in patients with Zika virus infection.[25]Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016 Apr 9;387(10027):1531-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5444521
http://www.ncbi.nlm.nih.gov/pubmed/26948433?tool=bestpractice.com
Neurologic consultation is recommended in patients with suspected GBS.[145]Gold CA, Josephson SA. Anticipating the challenges of Zika virus and the incidence of Guillain-Barré syndrome. JAMA Neurol. 2016 Aug 1;73(8):905-6.
http://archneur.jamanetwork.com/article.aspx?articleid=2526494
http://www.ncbi.nlm.nih.gov/pubmed/27272118?tool=bestpractice.com
An association between Zika infection and transient hearing loss has been reported in a small number of cases.[146]Vinhaes ES, Santos LA, Dias L, et al. Transient hearing loss in adults associated with Zika virus infection. Clin Infect Dis.2017 Mar 1;64(5):675-7.
http://cid.oxfordjournals.org/content/early/2016/12/05/cid.ciw770.full.pdf
http://www.ncbi.nlm.nih.gov/pubmed/27927858?tool=bestpractice.com
Congenital Zika syndrome is a recognized pattern of congenital anomalies (i.e., microcephaly, intracranial calcifications or other brain anomalies, or eye anomalies, among others) in infants associated with Zika virus infection during pregnancy.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
[3]França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016 Aug 27;388(10047):891-7.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)30902-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27372398?tool=bestpractice.com
[4]Melo AS, Aguiar RS, Amorim MM, et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol. 2016 Dec 1;73(12):1407-16.
http://jamanetwork.com/journals/jamaneurology/fullarticle/2557231
http://www.ncbi.nlm.nih.gov/pubmed/27695855?tool=bestpractice.com
[5]Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017 Mar 1;171(3):288-95.
https://jamanetwork.com/journals/jamapediatrics/fullarticle/2579543
http://www.ncbi.nlm.nih.gov/pubmed/27812690?tool=bestpractice.com
[6]Lucey D, Cummins H, Sholts S. Congenital Zika syndrome in 2017. JAMA. 2017 Apr 4;317(13):1368-9.
http://www.ncbi.nlm.nih.gov/pubmed/28384812?tool=bestpractice.com
Infants may present with microcephaly or other manifestations including spasticity, hypertonia, abnormal persistence of primitive reflexes, impaired cognitive development, delayed neuropsychomotor development, seizures, craniofacial disproportion, retrognathia, brainstem dysfunction, ocular abnormalities, hearing loss, speech disorders, impaired language development, alteration in tongue frenulum, absence of stapedial reflexes, findings on neuroimaging (e.g., cortical disorders, calcifications, ventriculomegaly), arthrogryposis (e.g., congenital joint contractures), low birth weight for gestational age, irritability, dysphagia, and feeding difficulties.[147]Leal MC, van der Linden V, Bezerra TP, et al. Characteristics of dysphagia in infants with microcephaly caused by congenital Zika virus infection, Brazil, 2015. Emerg Infect Dis. 2017 Aug;23(8):1253-9.
https://wwwnc.cdc.gov/eid/article/23/8/17-0354_article
http://www.ncbi.nlm.nih.gov/pubmed/28604336?tool=bestpractice.com
[148]Cristina da Silva Rosa B, Hernandez Alves Ribeiro César CP, Paranhos LR, et al. Speech-language disorders in children with congenital Zika virus syndrome: a systematic review. Int J Pediatr Otorhinolaryngol. 2020 Nov;138:110309.
http://www.ncbi.nlm.nih.gov/pubmed/32853874?tool=bestpractice.com
[149]Freitas DA, Souza-Santos R, Carvalho LMA, et al. Congenital Zika syndrome: a systematic review. PLoS One. 2020;15(12):e0242367.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7737899
http://www.ncbi.nlm.nih.gov/pubmed/33320867?tool=bestpractice.com
Features consistent with fetal immobility (e.g., dimples, feet malpositions, distal hand/finger contractures) may also be present.[150]Del Campo M, Feitosa IM, Ribeiro EM, et al; Zika Embryopathy Task Force-Brazilian Society of Medical Genetics ZETF-SBGM. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet A. 2017 Apr;173(4):841-57.
https://onlinelibrary.wiley.com/doi/10.1002/ajmg.a.38170
http://www.ncbi.nlm.nih.gov/pubmed/28328129?tool=bestpractice.com
Other presentations include ocular abnormalities in infants without microcephaly or other brain abnormalities, postpartum-onset microcephaly in infants born with a normal head circumference, postpartum-onset hydrocephalus in infants born with microcephaly, sleep electroencephalogram (EEG) abnormalities, and diaphragmatic paralysis in infants born with microcephaly and arthrogryposis.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
There have been reports of these abnormalities in infants who have a normal head circumference and with mothers who do not report having a rash during pregnancy.[3]França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016 Aug 27;388(10047):891-7.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)30902-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27372398?tool=bestpractice.com
[151]Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016 Aug 27;388(10047):898-904.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)30883-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27372395?tool=bestpractice.com
[152]Leal MC, Muniz LF, Caldas Neto SD, et al. Sensorineural hearing loss in a case of congenital Zika virus. Braz J Otorhinolaryngol. Jul-Aug 2020;86(4):513-5.
http://www.sciencedirect.com/science/article/pii/S1808869416301276
http://www.ncbi.nlm.nih.gov/pubmed/27444419?tool=bestpractice.com
[153]van der Linden V, Filho EL, Lins OG, et al. Congenital Zika syndrome with arthrogryposis: retrospective case series study. BMJ. 2016 Aug 9;354:i3899.
http://www.bmj.com/content/354/bmj.i3899.long
http://www.ncbi.nlm.nih.gov/pubmed/27509902?tool=bestpractice.com
[154]Leal MC, Muniz LF, Ferreira TS, et al. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection - Brazil, November 2015-May 2016. MMWR Morb Mortal Wkly Rep. 2016 Sep 2;65(34):917-9.
http://www.cdc.gov/mmwr/volumes/65/wr/mm6534e3.htm
http://www.ncbi.nlm.nih.gov/pubmed/27585248?tool=bestpractice.com
The syndrome does not appear to be associated with maternal disease severity.[155]Halai UA, Nielsen-Saines K, Moreira ME, et al. Maternal Zika virus disease severity, virus load, prior dengue antibodies and their relationship to birth outcomes. Clin Infect Dis. 2017 Sep 15;65(6):877-83.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5848222
http://www.ncbi.nlm.nih.gov/pubmed/28535184?tool=bestpractice.com
Although the risk appears to be greatest with infection in the first or second trimester, signs of congenital brain injury due to Zika virus infection acquired during the third trimester of pregnancy have been reported.[156]Soares de Souza A, Dias CM, Braga FD, et al. Fetal infection by Zika virus in the third trimester: report of 2 cases. Clin Infect Dis. 2016 Dec 15;63(12):1622-5.
https://academic.oup.com/cid/article/63/12/1622/2645588
http://www.ncbi.nlm.nih.gov/pubmed/27601223?tool=bestpractice.com
Poor head growth with microcephaly developing after birth has been reported in a small number of patients in Brazil.[157]van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth - Brazil. MMWR Morb Mortal Wkly Rep. 2016 Dec 2;65(47):1343-8.
https://www.cdc.gov/mmwr/volumes/65/wr/mm6547e2.htm
http://www.ncbi.nlm.nih.gov/pubmed/27906905?tool=bestpractice.com
Eye abnormalities may be the only initial finding; therefore, it is recommended that all infants with potential Zika virus exposure should undergo an eye exam regardless of the presence or absence of other symptoms.[158]Zin AA, Tsui I, Rossetto J, et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatr. 2017 Sep 1;171(9):847-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5710409
http://www.ncbi.nlm.nih.gov/pubmed/28715527?tool=bestpractice.com
Other infectious causes of microcephaly should be ruled out.[159]Devakumar D, Bamford A, Ferreira MU, et al. Infectious causes of microcephaly: epidemiology, pathogenesis, diagnosis, and management. Lancet Infect Dis. 2018 Jan;18(1):e1-13.
http://www.ncbi.nlm.nih.gov/pubmed/28844634?tool=bestpractice.com
Zika virus infection should be considered in an infant:[98]Centers for Disease Control and Prevention. Zika virus: clinical signs and symptoms of Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/clinical-signs/index.html
Case definitions
Case definitions have been published by WHO, CDC, and PAHO:
Case definitions vary and their sensitivity has been questioned, particularly in areas where other arboviruses, such as chikungunya and dengue, circulate. One study found that the symptoms most strongly associated with Zika virus infection included rash, pruritus, conjunctival hyperemia, absence of fever (axillary temperature <99.5°F [<37.5°C]), no petechiae, and no anorexia.[160]Braga JU, Bressan C, Dalvi APR, et al. Accuracy of Zika virus disease case definition during simultaneous dengue and chikungunya epidemics. PLoS One. 2017 Jun 26;12(6):e0179725.
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0179725
http://www.ncbi.nlm.nih.gov/pubmed/28650987?tool=bestpractice.com
See Criteria.
Investigations
Diagnosis can be confirmed with molecular testing, which includes reverse transcriptase-polymerase chain reaction (RT-PCR) for viral RNA. Serologic testing, which includes immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) plus plaque-reduction neutralization testing (PRNT) for Zika virus antibodies, may be recommended in some circumstances. PRNT can be performed to measure virus-specific neutralizing antibodies and discriminate between cross-reacting antibodies in primary flavivirus infections (e.g., dengue and yellow fever viruses, yellow fever vaccine recipients); however, there are currently few laboratories that perform this test.[161]Centers for Disease Control and Prevention. Zika virus: clinical testing and diagnosis for Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/diagnosis-testing/index.html
Suitable specimens include serum, plasma, whole blood, and urine.[162]World Health Organization. Laboratory testing for Zika virus and dengue virus infections: interim guidance. Jul 2022 [internet publication].
https://www.who.int/publications/i/item/WHO-ZIKV_DENV-LAB-2022.1
Availability of commercial tests depends on location.
Prenatal ultrasound and amniocentesis may be recommended in some pregnant women. Head circumference measurement, computed tomography/magnetic resonance imaging of the head, cranial ultrasound, hearing screen, ophthalmologic screen, and neurologic exam may be recommended in infants with suspected congenital Zika syndrome.
Nerve conduction studies/electromyography and cerebrospinal fluid (CSF) exam should be performed in patients with suspected GBS if available; however, these investigations are not needed to make a clinical diagnosis and should not delay treatment.[143]World Health Organization. Assesssment and management of Guillain-Barré syndrome in the context of Zika virus: interim guidance update. Aug 2016 [internet publication].
https://www.who.int/publications/i/item/WHO-ZIKV-MOC-16.4-Rev.1
Interpretation of these studies and definitive diagnosis requires consultation with a neurologist.
Limitations of testing
Molecular assays are the preferred detection method. However, the period of viral RNA detectability following infection is limited, with RNA less likely to be detectable after the first week following symptom onset.[162]World Health Organization. Laboratory testing for Zika virus and dengue virus infections: interim guidance. Jul 2022 [internet publication].
https://www.who.int/publications/i/item/WHO-ZIKV_DENV-LAB-2022.1
Serologic testing is not generally recommended to confirm the diagnosis. The duration of typical IgM detectability is about 12 weeks. However, data suggest that Zika virus IgM can persist beyond 12 weeks (for months or even years) in some people, and so a positive IgM result may not always indicate recent infection. Therefore, IgM test results cannot always reliably distinguish between infection that occurred before or during the current pregnancy, particularly in women with possible Zika exposure before the current pregnancy. Additionally, as the prevalence of Zika virus disease declines, the likelihood of false-positive test results increases.[163]Oduyebo T, Polen KD, Walke HT, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure - United States (including US territories), July 2017. MMWR Morb Mortal Wkly Rep. 2017 Jul 28;66(29):781-93.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6629e1.htm
http://www.ncbi.nlm.nih.gov/pubmed/28749921?tool=bestpractice.com
Serologic testing is also complicated by cross-reactivity with other flaviviruses, which may make conclusive determination of which flavivirus is responsible for the person’s recent infection difficult.[161]Centers for Disease Control and Prevention. Zika virus: clinical testing and diagnosis for Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/diagnosis-testing/index.html
If used, it should be done with careful consideration of the epidemiologic and clinical context.[162]World Health Organization. Laboratory testing for Zika virus and dengue virus infections: interim guidance. Jul 2022 [internet publication].
https://www.who.int/publications/i/item/WHO-ZIKV_DENV-LAB-2022.1
Sensitivity and negative predictive value for all Zika investigations has not been fully established, especially for asymptomatic people. Consequently, transmission prevention precautions remain important even when a test is negative.
Testing a person’s blood, urine, or genital secretions to determine their risk of sexually transmitting Zika virus is not recommended.[164]Centers for Disease Control and Prevention. Zika virus: sexual transmission of Zika virus. Aug 2024 [internet publication].
https://www.cdc.gov/zika/hcp/sexual-transmission/index.html
Testing recommendations
Testing recommendations may vary between countries, and depend on the current epidemiologic situation. Consult your local guidance. The testing recommendations in this topic are mainly based on CDC guidance. Testing recommendations may differ between guidelines and locations, with WHO and PAHO also offering recommendations:
Zika and dengue virus infections have a similar global geographic distribution, and the clinical presentation is similar. Therefore, patients with suspected Zika virus infection should always be evaluated for possible dengue virus infection.[161]Centers for Disease Control and Prevention. Zika virus: clinical testing and diagnosis for Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/diagnosis-testing/index.html
[165]Sharp TM, Fischer M, Muñoz-Jordán JL, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep. 2019 Jun 14;68(1):1-10.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6581290
http://www.ncbi.nlm.nih.gov/pubmed/31194720?tool=bestpractice.com
Testing in nonpregnant individuals
Asymptomatic individuals
Symptomatic individuals
Testing for Zika and dengue virus infection is not currently recommended for people living in or with recent travel to the US and its territories. However, testing is recommended in people living in or with recent travel to an area with an active CDC Zika travel health notice, or to an area with current or past Zika transmission outside of the US and its territories.[161]Centers for Disease Control and Prevention. Zika virus: clinical testing and diagnosis for Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/diagnosis-testing/index.html
If testing is recommended, Zika and dengue RT-PCR should be performed on serum collected ≤7 days after symptom onset. A positive result typically provides evidence of acute infection. Zika and dengue IgM antibody testing should be performed on RT-PCR-negative serum specimens and serum collected >7 days after onset of symptoms. If either Zika or dengue IgM antibody testing is positive, PRNT testing should be performed against Zika, dengue, and other flaviviruses endemic to the region where exposure occurred to confirm the diagnosis (if confirmation is required).
Testing in pregnant women
The current CDC recommendations for testing pregnant women in the context of low to no Zika virus transmission globally are detailed below.[161]Centers for Disease Control and Prevention. Zika virus: clinical testing and diagnosis for Zika virus disease. May 2024 [internet publication].
https://www.cdc.gov/zika/hcp/diagnosis-testing/index.html
Asymptomatic pregnant women
Routine testing is not currently recommended for asymptomatic pregnant women living in or with recent travel to the US and its territories.
Routine testing is also not recommended for pregnant women who have traveled to an area with current or past Zika virus transmission outside the US and its territories during pregnancy.
However, RT-PCR may be considered in pregnant women with recent travel to an area with an active Zika travel health notice during pregnancy, up to 12 weeks after travel.
Symptomatic pregnant women
Pregnant women with prenatal ultrasound findings consistent with congenital Zika virus infection
Women who lived in or traveled to an area with an active CDC Zika travel health notice or current or past Zika virus transmission during pregnancy, or had sex with someone who lived in or travel to an area with an active CDC Zika travel health notice or current or past Zika virus transmission during pregnancy should have Zika virus RT-PCR and serology performed on maternal serum, and RT-PCR on maternal urine. If Zika RT-PCR results are negative and the serology is positive, confirmatory PRNT testing should be performed for Zika and dengue.
Pregnant women with laboratory evidence of possible Zika virus infection should have serial ultrasounds every 3 to 4 weeks to monitor fetal anatomy and growth, and be referred to a specialist.[163]Oduyebo T, Polen KD, Walke HT, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure - United States (including US territories), July 2017. MMWR Morb Mortal Wkly Rep. 2017 Jul 28;66(29):781-93.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6629e1.htm
http://www.ncbi.nlm.nih.gov/pubmed/28749921?tool=bestpractice.com
Intrauterine diagnosis of microcephaly is made when the head circumference is ≥2 standard deviations below the mean for gender and gestational age.[166]Pan American Health Organization. Preliminary guidelines for the surveillance of microcephaly in newborns in settings with risk of Zika virus circulation. 2016 [internet publication].
http://iris.paho.org/xmlui/handle/123456789/18601
The CDC provide the following resources to help determine whether patients meet the epidemiologic criteria required for testing:
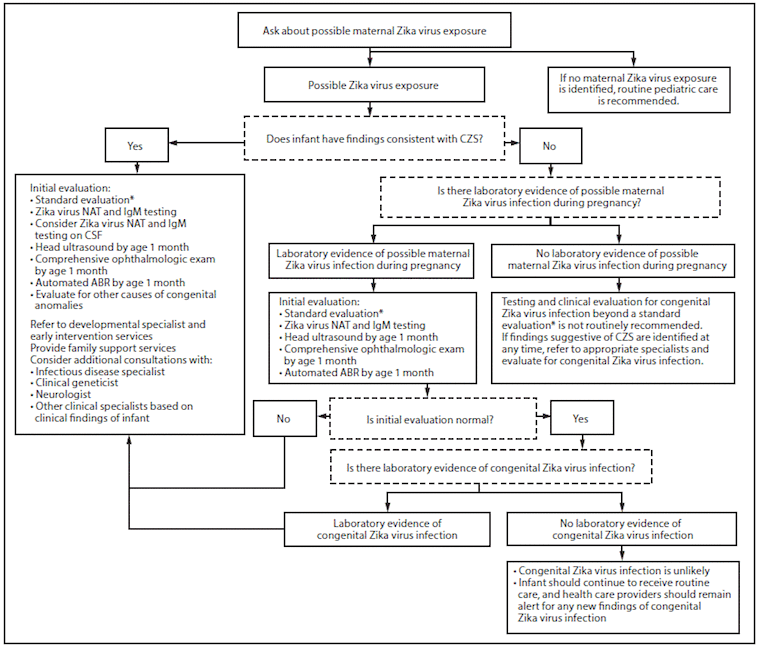
Testing in infants with suspected congenital Zika syndrome
Congenital Zika syndrome is a recognized pattern of congenital anomalies (i.e., microcephaly, intracranial calcifications or other brain anomalies, or eye anomalies, among others) in infants associated with Zika virus infection during pregnancy.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
[3]França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016 Aug 27;388(10047):891-7.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)30902-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27372398?tool=bestpractice.com
[4]Melo AS, Aguiar RS, Amorim MM, et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol. 2016 Dec 1;73(12):1407-16.
http://jamanetwork.com/journals/jamaneurology/fullarticle/2557231
http://www.ncbi.nlm.nih.gov/pubmed/27695855?tool=bestpractice.com
[5]Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017 Mar 1;171(3):288-95.
https://jamanetwork.com/journals/jamapediatrics/fullarticle/2579543
http://www.ncbi.nlm.nih.gov/pubmed/27812690?tool=bestpractice.com
[6]Lucey D, Cummins H, Sholts S. Congenital Zika syndrome in 2017. JAMA. 2017 Apr 4;317(13):1368-9.
http://www.ncbi.nlm.nih.gov/pubmed/28384812?tool=bestpractice.com
Other presentations include ocular abnormalities in infants without microcephaly or other brain abnormalities, postpartum-onset microcephaly in infants born with a normal head circumference, postpartum-onset hydrocephalus in infants born with microcephaly, sleep EEG abnormalities, and diaphragmatic paralysis in infants born with microcephaly and arthrogryposis.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
[167]van der Linden V, de Lima Petribu NC, Pessoa A, et al. Association of severe hydrocephalus with congenital Zika syndrome. JAMA Neurol. 2019 Feb 1;76(2):203-10.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6439957
http://www.ncbi.nlm.nih.gov/pubmed/30452526?tool=bestpractice.com
Suspected cases should be referred to a pediatrician.
Both molecular and serologic testing are recommended in:[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
Infants with clinical findings consistent with congenital Zika syndrome born to mothers with possible Zika virus exposure in pregnancy, regardless of maternal testing results
Infants without clinical findings consistent with congenital Zika syndrome who were born to mothers with laboratory evidence of possible Zika virus infection.
Laboratory testing is not routinely recommended for infants without clinical findings consistent with congenital Zika syndrome born to mothers with possible Zika exposure during pregnancy but without laboratory evidence of maternal infection.
Initial samples for testing (i.e., infant serum and urine; whole blood; cord blood is no longer recommended) should be collected directly from the infant in the first 2 days of life for simultaneous RT-PCR (on serum and urine) and IgM ELISA testing (note: specimens collected within the first few weeks to months after birth still may be useful, especially in infants born in areas without risk of Zika). CSF testing can be considered if CSF is obtained for other purposes.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
A positive RT-PCR on serum or urine confirms congenital infection. A negative RT-PCR result with a positive IgM result suggests probable congenital infection.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
PRNT can be used to help identify false-positive results, and confirm or rule out congenital Zika virus infection in children ages ≥18 months. [Figure caption and citation for the preceding image starts]: Interpretation of results of laboratory testing for evidence of congenital Zika virus infectionCenters for Disease Control and Prevention [Citation ends].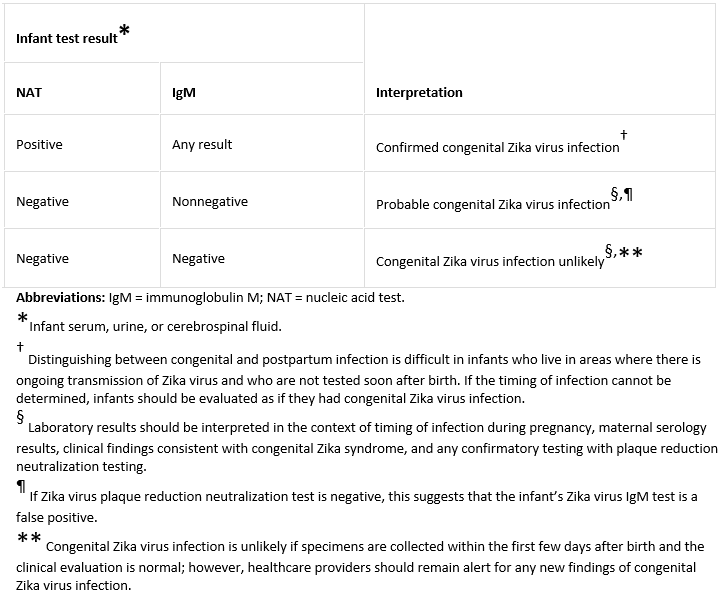
A standard evaluation is recommended in all infants born to mothers with possible or confirmed infection, regardless of whether the infant has clinical findings consistent with congenital Zika syndrome. The evaluation should include:[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
In addition to this, infants with clinical findings consistent with congenital Zika syndrome, and infants without clinical findings born to mothers with laboratory evidence of possible Zika virus infection should have the following investigations:[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
Infants with clinical findings consistent with congenital Zika syndrome should also have the following:[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
Consultation with infectious disease, clinical genetics, and neurology specialists, as well as any other clinical specialists based on the infant’s clinical findings (e.g., endocrinologist, lactation specialist, gastroenterologist, speech or occupational therapist, orthopedist, physical therapist, pulmonologist).
Infants who do not have clinical findings consistent with congenital Zika syndrome should be referred to an appropriate specialist if any clinical findings are noted at follow-up visits.
Follow-up care requires a multidisciplinary team to facilitate coordination of care and will depend on the clinical findings in the infant. Healthcare providers should be vigilant for other clinical findings (e.g., difficulty swallowing, hydrocephaly) or new clinical findings. A standard evaluation should be performed at subsequent well-child visits in all infants, along with routine pediatric care. Automated ABR testing is no longer recommended in infants at 4 to 6 months of age if they passed the initial hearing screen with ABR.[2]Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017 Oct 20;66(41):1089-99.
https://www.cdc.gov/mmwr/volumes/66/wr/mm6641a1.htm?s_cid=mm6641a1_w
http://www.ncbi.nlm.nih.gov/pubmed/29049277?tool=bestpractice.com
The WHO offers specific guidance for the screening, assessment, and management of neonates and infants with congenital Zika syndrome.
WHO: screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero
Opens in new window
[Figure caption and citation for the preceding image starts]: Recommended Zika virus testing and evaluation of infants born to mothers with laboratory evidence of Zika virus infection during pregnancyCenters for Disease Control and Prevention [Citation ends].