Etiology
Sepsis is the systemic response to infection. The primary etiology can therefore be attributed to both the infecting pathogen and the host response. While any infection may precipitate sepsis, the most common pathogens are bacteria, viruses, or fungi. The type of pathogen varies according to a number of host factors, including age, comorbidity, and geographic location. Typical/important pathogens by patient group are listed below.
Early-onset neonatal sepsis (EOS):[9][20][21][22]
Defined as neonatal sepsis occurring in the first 72 hours of life.
Group B streptococci and gram-negative bacilli (especially Escherichia coli) are by far the most common causative pathogens in EOS.
Staphylococcus aureus and coagulase-negative staphylococci, Haemophilus influenzae, and enterococci make up most of the rest of the bacterial etiologies in EOS.
Listeria monocytogenes is a rare infection, but has a disproportionate proclivity to infect pregnant women and their fetuses. It may cause EOS or late-onset neonatal sepsis.
Late-onset neonatal sepsis (LOS):[10][20][23]
Defined as neonatal sepsis occurring after the first 72 hours to 1 month of life.
Coagulase-negative staphylococci are the most common cause of LOS due to the high incidence in vascular-catheter-associated infection in hospitalized neonatal patients.
May also be caused by the same organisms responsible for EOS.
Infants and young children:[4][14][24]
Streptococcus pneumoniae remains a major cause of invasive bacterial infection in childhood.
Neisseria meningitidis occurs in a bimodal age distribution affecting young children and adolescents. It is less common since uptake of vaccination.
S aureus and group A streptococci both may cause sepsis in previously well children. Higher than normal notifications of scarlet fever and invasive group A streptococcus (iGAS) disease were reported in England in the season 2022-2023.[25]
H influenzae type b is an important cause of sepsis worldwide, but rare in the developed world due to vaccination.
Bordetella pertussis, although rare, may cause a severe illness in young infants prior to primary vaccination.
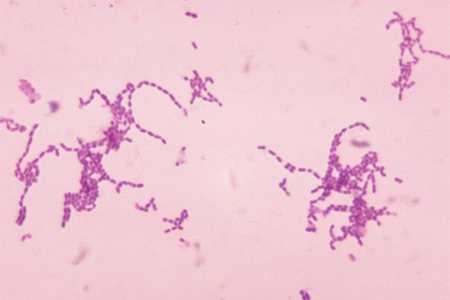
Data on specific infecting organisms in resource-poor settings are less robust, but diarrhea and pneumonia are the most common infections (and causes of death).[Figure caption and citation for the preceding image starts]: Photomicrograph of Gram-stained Streptococcus species bacteriaImage provided by the US Centers for Disease Control and Prevention Public Health Image Library [Citation ends].
Hospitalized infants and children:[26]
Cause of hospital-acquired infection will depend on local bacterial epidemiology
Coagulase-negative staphylococci are usually associated with vascular catheter infection
Methicillin-resistant S aureus is more common in the US compared with the UK
Gram-negative organisms such as Pseudomonas aeruginosa, Klebsiella, E coli, and Acinetobacter species.
Asplenic or functional asplenia:[27][28]
Salmonella sepsis, including Salmonella osteomyelitis in sickle cell disease
Other encapsulated organisms (e.g., S pneumoniae, H influenzae).
Mosquito-borne and other tropical diseases:[29]
Malaria (Plasmodium falciparum), dengue virus, and Burkholderia pseudomallei (melioidosis) are important causes of sepsis in endemic areas.
Other organisms:[15]
Fungal (e.g., Candida species, Aspergillus species) and viral (e.g., influenza, respiratory syncytial virus, human metapneumovirus, varicella, and herpes simplex virus) pathogens account for up to 5.3% and 2.9% of sepsis in children, respectively, in the US.
Pathophysiology
The normal host response to infection is an inflammatory process, aimed at localizing and controlling the infection. The inflammatory response is triggered when innate immune cells (e.g., macrophages) recognize the invading pathogen. For example, lipopolysaccharides of gram-negative bacteria are recognized by receptors on innate immune cells.[30] Following binding to these sites, the immune cells are activated to secrete pro-inflammatory cytokines, which are responsible for recruiting polymorphonuclear cells to the site of infection. These polymorphonuclear cells also release pro-inflammatory cytokines leading to vasodilation and vascular permeability (capillary leak).[30] In the normal host response, this pro-inflammatory response is regulated and localized by a simultaneous anti-inflammatory response.[30] Sepsis occurs when this normal, pro-inflammatory host response exceeds its usual homeostatic constraints and becomes a generalized process, resulting in inflammation remote from the infection source.
While this model of sepsis is intuitive, the results of emerging research suggest that it may be an oversimplification. The pathophysiology may include processes such as endothelium dysfunction, cell death, bioenergetic derangement, and immunoparalysis.
Endothelium dysfunction:
The endothelium lines the entire cardiovascular system and is responsible for various physiologic responses (e.g., control of vascular tone and regulation of coagulation) to maintain appropriate blood flow and oxygen delivery to organs. Disruptions and aberrations of endothelial function underpin many of the cardiovascular manifestations of sepsis.[30] Inflammatory cytokines and effector cells (e.g., neutrophils) engage and interact with the endothelium during sepsis; the resultant activated endothelium then executes many of the systemic effects. These changes include widespread capillary leak (i.e., increased capillary permeability), which in turn leads to tissue edema and a reduction in circulating volume. This may lead to, or exacerbate, shock (due to hypovolemia) and organ dysfunction.[30] The endothelium is responsible for releasing nitric oxide, a potent vasodilator. The role of nitric oxide in normal health is to regulate regional blood flow according to changes in demand. In sepsis, the damaged, activated endothelium may release large amounts of nitric oxide systemically, resulting in widespread vasodilation and decreased vascular resistance.[31] Coagulation is frequently deranged in sepsis owing to activation of the coagulation pathways by the activated endothelium.[30] The typical picture is disseminated intravascular coagulation (i.e., platelet consumption and prolonged clotting). The clinical manifestation is a combination of ischemic organ dysfunction due to intravascular clotting, and bleeding.
In addition to the microcirculatory effects of sepsis, a direct effect on the heart from circulating mediators may reduce the performance of the myocardium, contributing to decreased cardiac output and shock.[32]
Cell death:
The precise nature of cell death in sepsis is an area of ongoing research, and requires further delineation. In broad terms, cell death in sepsis occurs owing to a combination of necrosis and apoptosis. The precise contribution of each of these mechanisms to cell death in sepsis is debated.[33]
Aberrations of apoptotic mechanisms have been described in several cell types in sepsis. Delayed apoptosis in innate immune cells (e.g., neutrophils and macrophages) may result in delayed resolution of pro-inflammatory responses. Conversely, increased rates of apoptosis in lymphocytes have been described in septic patients, which may result in an acquired immunosuppression.[33]
Bioenergetic derangement:
Organ failure in sepsis is not entirely attributable to the circulatory effects described above. Another characteristic of septic shock is widespread bioenergetic failure resulting from a reduced cellular consumption of oxygen. This may be a result of direct inhibition of mitochondrial function (i.e., respiration) by inflammatory mediators such as nitric oxide.[34]
The origin of this response is unclear, but it has been proposed that mitochondrial dysfunction and the resulting energy failure in sepsis is an adaptive response.[35]
Immunoparalysis:
In addition to the systemic pro-inflammatory response, the host response in sepsis also includes a concomitant, anti-inflammatory response. This is referred to as the compensatory anti-inflammatory response syndrome.[36]
The evolutionary provenance of this response may be as a counter-regulatory, or adaptive, response to constrain and control the pro-inflammatory response.[37]
A putative effect of compensatory anti-inflammatory response syndrome is a modulation of the host immune system, known as immunoparalysis, which may render the host more susceptible to secondary infections.[38][39]
Data suggest that there is significant heterogeneity in patients' host responses. This heterogeneity applies to the balance of pro- and anti-inflammatory processes, and the nature of humoral and cellular changes.[40]
Classification
Classification of neonatal sepsis
Neonatal sepsis is defined as a clinical syndrome of sepsis, and/or isolation of a pathogen in the blood stream, in an infant in the first 28 days of life.[8] Symptoms and clinical signs are often less apparent or more subtle than in older children. Sepsis in newborns is usually classified in terms of timing of onset in relation to birth:
Early-onset neonatal sepsis: neonatal sepsis occurring in the first 72 hours of life[9]
Late-onset neonatal sepsis: neonatal sepsis occurring after the first 72 hours of life.[10]
Classification by age group
For the purposes of consistent classification, the following age groups are used for referencing normal ranges of physiologic variables and laboratory values:[3]
Newborn: 0 days to 1 week
Neonate: 0 days to 1 month
Infant: 1 month to <2 years
Toddler and preschool: ≥2 years to <6 years
School-age child: ≥6 years to <13 years
Adolescent and young adult: ≥13 years to <18 years.
Note that preterm infants are not classified in this age scheme.
Use of this content is subject to our disclaimer