Increased public awareness of breast cancer has led to a significant proportion of women presenting to clinicians with palpable masses. Breast cancers that are detected clinically or by breast self-exam are typically of more advanced stage.[31]Guth U, Huang DJ, Huber M, et al. Tumor size and detection in breast cancer: self-examination and clinical breast examination are at their limit. Cancer Detect Prev. 2008;32(3):224-8.
http://www.ncbi.nlm.nih.gov/pubmed/18790576?tool=bestpractice.com
Masses identified on mammography may undergo further evaluation with ultrasonography to determine whether they are cystic or solid. Mammographic screening has led to more breast cancers being detected at a nonpalpable stage.[32]Breen N, Yabroff KR, Meissner HI. What proportion of breast cancers are detected by mammography in the United States? Cancer Detect Prev. 2007;31(3):220-4.
http://www.ncbi.nlm.nih.gov/pubmed/17573202?tool=bestpractice.com
[33]Benson JR, Jatoi I, Keisch M, et al. Early breast cancer. Lancet. 2009 Apr 25;373(9673):1463-79.
http://www.ncbi.nlm.nih.gov/pubmed/19394537?tool=bestpractice.com
History
The median age at breast cancer diagnosis in women is 63 years.[34]National Cancer Institute. Cancer stat facts: female breast cancer. 2024 [internet publication].
https://seer.cancer.gov/statfacts/html/breast.html
The majority of breast cancers are sporadic (i.e., in patients without a family history of breast cancer).
Approximately 5% to 10% of all breast cancers are diagnosed in patients with a mutation in the BRCA-1 or BRCA-2 genes.[35]Lynch HT, Silva E, Snyder C, et al. Hereditary breast cancer - part I: diagnosing hereditary breast cancer syndromes. Breast J. 2008 Jan-Feb;14(1):3-13.
http://www.ncbi.nlm.nih.gov/pubmed/18086272?tool=bestpractice.com
Pathogenic variants in BRCA-1 and BRCA-2 are associated with a high risk of breast cancer, with odds ratios of 7.62 (95% CI, 5.33 to 11.27) and 5.23 (95% CI, 4.09 to 6.77), respectively.[36]Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021 Feb 4;384(5):440-51.
https://www.nejm.org/doi/10.1056/NEJMoa2005936
http://www.ncbi.nlm.nih.gov/pubmed/33471974?tool=bestpractice.com
BRCA-1 and BRCA-2 mutations are more common in women with a family history of: breast cancer before age 50 years, bilateral breast cancer, breast and ovarian cancer in the same relative, male breast cancer, multiple cases of breast cancer in the family, one or more family members with 2 primary types of BRCA-related cancer (e.g. ovarian cancer) and Ashkenazi Jewish ancestry.[37]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: colorectal, endometrial, and gastric [internet publication].
https://www.nccn.org/guidelines/category_2
Therefore family history should include any family members with cancer, their primary cancer site, whether the affected relative had multiple primary cancers, age at diagnosis, age at death and sex.
US Preventive Services Task Force. BRCA-related cancer: risk assessment, genetic counselling and genetic testing. 2019
Opens in new window
Prior biopsy history of atypical hyperplasia carries a four- to fivefold increase in the risk of developing breast cancer.[38]Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985 Jan 17;312(3):146-51.
http://www.ncbi.nlm.nih.gov/pubmed/3965932?tool=bestpractice.com
[39]Marshall LM, Hunter DJ, Connolly JL, et al. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997 May;6(5):297-301.
http://cebp.aacrjournals.org/content/6/5/297.full.pdf+html
http://www.ncbi.nlm.nih.gov/pubmed/9149887?tool=bestpractice.com
For those with a history of lobular carcinoma in situ (LCIS), there is a seven- to 12-fold increase in risk.[40]Sakorafas GH, Krespis E, Pavlakis G. Risk estimation for breast cancer development; a clinical perspective. Surg Oncol. 2002 May;10(4):183-92.
http://www.ncbi.nlm.nih.gov/pubmed/12020673?tool=bestpractice.com
Patients diagnosed with an invasive cancer have a risk of contralateral breast cancer that is estimated at 0.5% to 1% a year, cumulative over their lifetime.[40]Sakorafas GH, Krespis E, Pavlakis G. Risk estimation for breast cancer development; a clinical perspective. Surg Oncol. 2002 May;10(4):183-92.
http://www.ncbi.nlm.nih.gov/pubmed/12020673?tool=bestpractice.com
In postmenopausal women, hormone replacement therapy with an estrogen alone is associated with little or no change in the risk of breast cancer.[41]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng23
An estrogen prescribed in combination with a progestin is associated with an increase in the incidence of breast cancer.[41]National Institute for Health and Care Excellence. Menopause: diagnosis and management. Nov 2024 [internet publication].
https://www.nice.org.uk/guidance/ng23
[42]de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric. 2016 Aug;19(4):313-5.
http://www.ncbi.nlm.nih.gov/pubmed/27322027?tool=bestpractice.com
[43]Ross RK, Paganini-Hill A, Wan PC, et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000 Feb 16;92(4):328-32.
http://jnci.oxfordjournals.org/content/92/4/328.full
http://www.ncbi.nlm.nih.gov/pubmed/10675382?tool=bestpractice.com
[44]Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321-33.
https://jamanetwork.com/journals/jama/fullarticle/195120
http://www.ncbi.nlm.nih.gov/pubmed/12117397?tool=bestpractice.com
[45]Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019 Sep 28;394(10204):1159-68.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31709-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31474332?tool=bestpractice.com
[46]Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women's Health Initiative randomized clinical trials. JAMA. 2020 Jul 28;324(4):369-80.
https://jamanetwork.com/journals/jama/fullarticle/2768806
http://www.ncbi.nlm.nih.gov/pubmed/32721007?tool=bestpractice.com
There is an association between increased breast density (categorized by Breast Imaging Reporting and Data System [BI-RADS]) and breast cancer incidence in women over the age of 65 years; however, the mechanisms underlying the observed association are not yet clear.[47]Advani SM, Zhu W, Demb J, et al. Association of breast density with breast cancer risk among women aged 65 years or older by age group and body mass index. JAMA Netw Open. 2021 Aug 2;4(8):e2122810.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2783508
http://www.ncbi.nlm.nih.gov/pubmed/34436608?tool=bestpractice.com
Physical exam
Findings on physical exam alone cannot definitively establish a mass as benign or malignant. However, irregular fixed masses are suspicious for malignancy.[48]Shapley M, Mansell G, Jordan JL, et al. Positive predictive values of ≥5% in primary care for cancer: systematic review. Br J Gen Pract. 2010 Sep;60(578):e366-77.
http://www.ncbi.nlm.nih.gov/pubmed/20849687?tool=bestpractice.com
Malignant lesions may also be associated with skin thickening (e.g., peau d'orange) or nipple changes.[3]Practice bulletin no. 164: diagnosis and management of benign breast disorders. Obstet Gynecol. 2016 Jun;127(6):e141-56.
http://www.ncbi.nlm.nih.gov/pubmed/27214189?tool=bestpractice.com
A complete bilateral breast exam including assessment of the axillae and regional lymph nodes, should be performed to look for:[49]Hindle WH. Breast mass evaluation. Clin Obstet Gynecol. 2002 Sep;45(3):750-7.
http://www.ncbi.nlm.nih.gov/pubmed/12370615?tool=bestpractice.com
Variation in breast size[Figure caption and citation for the preceding image starts]: Patient with inflammatory breast cancer who presented with a shrinking breastCourtesy of Dr Anees Chagpar [Citation ends].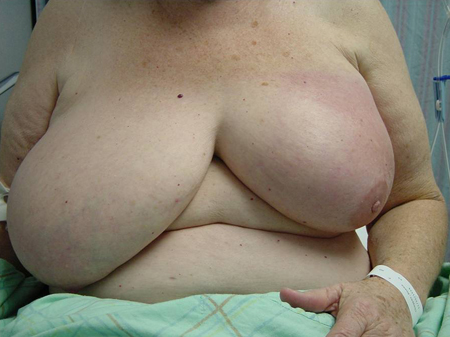
Fungating masses[Figure caption and citation for the preceding image starts]: Obvious mass with skin involvement on right breastCourtesy of Dr Anees Chagpar [Citation ends]. [Figure caption and citation for the preceding image starts]: Obvious mass with skin involvement on left breastCourtesy of Dr Anees Chagpar [Citation ends].
[Figure caption and citation for the preceding image starts]: Obvious mass with skin involvement on left breastCourtesy of Dr Anees Chagpar [Citation ends].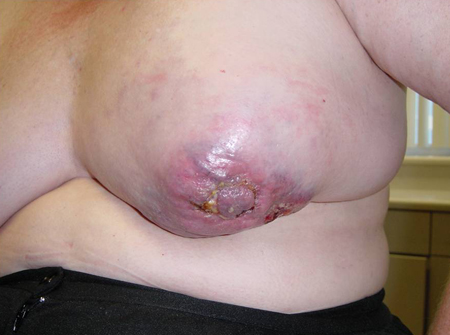
Dimpling or retraction of the skin[Figure caption and citation for the preceding image starts]: Patient with large breast mass and retraction at 6 o'clock of left breast, noted on elevating armsCourtesy of Dr Anees Chagpar [Citation ends].
Nipple inversion or excoriation (classic finding of Paget disease of the breast).[Figure caption and citation for the preceding image starts]: Excoriation of the nipple in a patient with Paget diseaseCourtesy of Dr Anees Chagpar [Citation ends].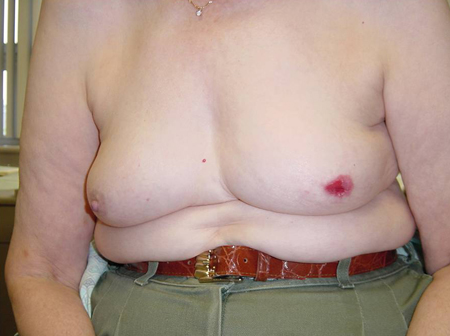
These findings may be accentuated by having the patient stretch her arms over her head. Similarly, having patients place their hands on their hips and squeeze inward while flexing the pectoral muscles may reveal chest wall involvement.
The lymph nodes draining the cervical, supraclavicular, and infraclavicular fossae should be evaluated. A comprehensive exam necessitates evaluation of the patient both seated upright and lying supine, as masses are often more readily appreciated in one position than the other.
One randomized controlled trial found that encouraging documentation of the physical exam using a dedicated form resulted in a higher rate of further evaluation of breast masses and an improved cancer detection rate.[50]Goodson WH 3rd, Hunt TK, Plotnik JN, et al. Optimization of clinical breast examination. Am J Med. 2010 Apr;123(4):329-34.
http://www.ncbi.nlm.nih.gov/pubmed/20362752?tool=bestpractice.com
These results indicate that a focused physical exam can result in performance improvement.
Mammography
US guidelines recommend that all women ≥30 years old presenting with a breast mass should have a diagnostic mammogram (with additional views such as spot compression, magnification, or tangential) and digital breast tomosynthesis (or contrast-enhanced mammography if available) plus an ultrasound.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[51]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
Multifocal or multicentric disease should be noted.
In the setting of a palpable breast mass, mammography is 82% to 94% sensitive and 55% to 84% specific for detecting breast cancer.[52]Malur S, Wurdinger S, Moritz A, et al. Comparison of written reports of mammography, sonography and magnetic resonance mammography for preoperative evaluation of breast lesions, with special emphasis on magnetic resonance mammography. Breast Cancer Res. 2001;3(1):55-60.
https://www.doi.org/10.1186/bcr271
http://www.ncbi.nlm.nih.gov/pubmed/11250746?tool=bestpractice.com
[53]Kerlikowske K, Grady D, Rubin SM, et al. Efficacy of screening mammography: a meta-analysis. JAMA. 1995 Jan 11;273(2):149-54.
http://www.ncbi.nlm.nih.gov/pubmed/7799496?tool=bestpractice.com
[54]Kacl GM, Liu P, Debatin JF, et al. Detection of breast cancer with conventional mammography and contrast-enhanced MR imaging. Eur Radiol. 1998;8(2):194-200.
http://www.ncbi.nlm.nih.gov/pubmed/9477265?tool=bestpractice.com
[55]Bone B, Pentek Z, Perbeck L, et al. Diagnostic accuracy of mammography and contrast-enhanced MR imaging in 238 histologically verified breast lesions. Acta Radiol. 1997 Jul;38(4 pt 1):489-96.
http://www.ncbi.nlm.nih.gov/pubmed/9240665?tool=bestpractice.com
In women <30 years with a palpable breast mass and findings on ultrasound that are suspicious for malignancy, US guidelines recommend mammography and digital breast tomography as subsequent imaging studies.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[51]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
Radiologists often characterize the findings on ultrasound or mammography according to the BI-RADS.[56]Kerlikowske K, Smith-Bindman R, Ljung BM, et al. Evaluation of abnormal mammography results and palpable breast abnormalities. Ann Intern Med. 2003 Aug 19;139(4):274-84.
http://www.ncbi.nlm.nih.gov/pubmed/12965983?tool=bestpractice.com
[57]American College of Radiology. Breast imaging reporting & data system (BI-RADS®). 2013 [internet publication].
https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
Some have advocated recording breast density and hormone therapy use, as these significantly affect mammography performance.[58]Cox B, Ballard-Barbash R, Broeders M, et al; International Cancer Screening Network. Recording of hormone therapy and breast density in breast screening programs: summary and recommendations of the International Cancer Screening Network. Breast Cancer Res Treat. 2010 Dec;124(3):793-800.
http://www.ncbi.nlm.nih.gov/pubmed/20414718?tool=bestpractice.com
[59]Woods RW, Sisney GS, Salkowski LR, et al. The mammographic density of a mass is a significant predictor of breast cancer. Radiology. 2011 Feb;258(2):417-25.
http://www.ncbi.nlm.nih.gov/pubmed/21177388?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Breast Imaging Reporting and Data System (BIRADS) criteriaCourtesy of Dr Anees Chagpar [Citation ends].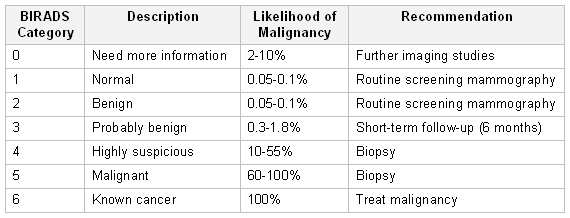 [Figure caption and citation for the preceding image starts]: Magnification view demonstrating irregular spiculated mass with associated calcificationsCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Magnification view demonstrating irregular spiculated mass with associated calcificationsCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends].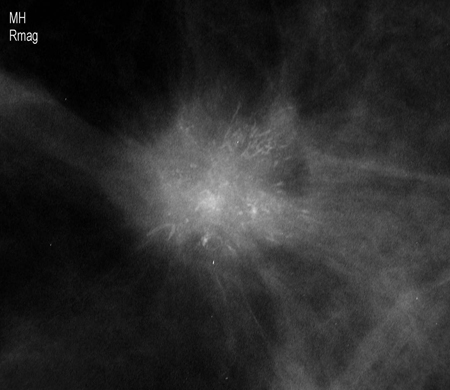 [Figure caption and citation for the preceding image starts]: Screening mammogram demonstrating breast massCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Screening mammogram demonstrating breast massCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends].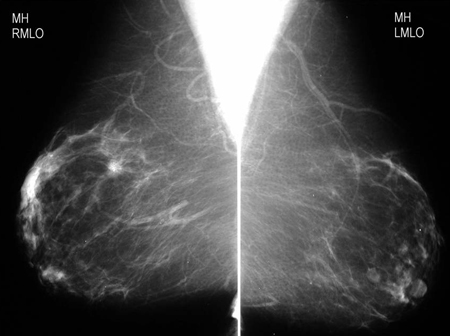
BIRADS was developed by the American College of Radiologists as a standard of comparison for rating mammograms and breast ultrasound images.[57]American College of Radiology. Breast imaging reporting & data system (BI-RADS®). 2013 [internet publication].
https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
It sets up a classification for level of suspicion (LOS) for the possibility of breast cancer. A score of 1 to 2 allows the patient to resume routine screening; a score of 3 may require further imaging with mammography and/or ultrasound scan, or short-term follow-up; a score of 4 to 5 requires a tissue biopsy.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[57]American College of Radiology. Breast imaging reporting & data system (BI-RADS®). 2013 [internet publication].
https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
A negative imaging study of a palpable mass also requires surgical follow-up. A score of 6 is given only after a biopsy has been examined and found to be cancerous, in which case treatment is required.[57]American College of Radiology. Breast imaging reporting & data system (BI-RADS®). 2013 [internet publication].
https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
Ultrasound of the breast
Ultrasonography is often considered the initial diagnostic test of choice in patients <30 years old, because the density of breast tissue in younger women limits the sensitivity of mammography.[3]Practice bulletin no. 164: diagnosis and management of benign breast disorders. Obstet Gynecol. 2016 Jun;127(6):e141-56.
http://www.ncbi.nlm.nih.gov/pubmed/27214189?tool=bestpractice.com
[51]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
[60]American College of Radiology. ACR practice parameter for the performance of a diagnostic breast ultrasound examination. 2021 [internet publication].
https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-Breast.pdf?la=en
[61]Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002 Oct;225(1):165-75.
http://pubs.rsna.org/doi/full/10.1148/radiol.2251011667
http://www.ncbi.nlm.nih.gov/pubmed/12355001?tool=bestpractice.com
The false-negative rate for mammography has been reported to be as high as 52% in patients <35 years old with a palpable malignant breast mass.[62]Max MH, Klamer TW. Breast cancer in 120 women under 35 years old: a 10-year community-wide survey. Am Surg. 1984 Jan;50(1):23-5.
http://www.ncbi.nlm.nih.gov/pubmed/6691629?tool=bestpractice.com
Ultrasound is usually performed in addition to mammography in the assessment of older women with suspected breast cancer.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
[63]Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023 Dec 8 [Epub ahead of print].
https://www.annalsofoncology.org/article/S0923-7534(23)05104-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38101773?tool=bestpractice.com
Ultrasound may be used as the initial diagnostic test in women aged 30-39 years where a simple cyst is suspected and the risk of breast cancer is low.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
Ultrasound is routinely available in the outpatient setting and is a ready extension of the physical exam. The American College of Radiology has published guidelines that may aid physicians in the performance of breast ultrasound.[60]American College of Radiology. ACR practice parameter for the performance of a diagnostic breast ultrasound examination. 2021 [internet publication].
https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-Breast.pdf?la=en
[64]American College of Radiology. ACR practice parameter for the performance of ultrasound-guided percutaneous breast interventional procedures. 2021 [internet publication].
https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-GuidedBreast.pdf?la=en
Ultrasound may identify simple or complex cyst architecture.[51]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
Simple cysts are smooth, round, well-demarcated, fluid-filled lesions, and are anechoic. If they have no internal septations or debris, they may simply be followed. Ultrasound is not able to detect microcalcifications in the breast.[Figure caption and citation for the preceding image starts]: Ultrasonographic image of a simple cystCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends].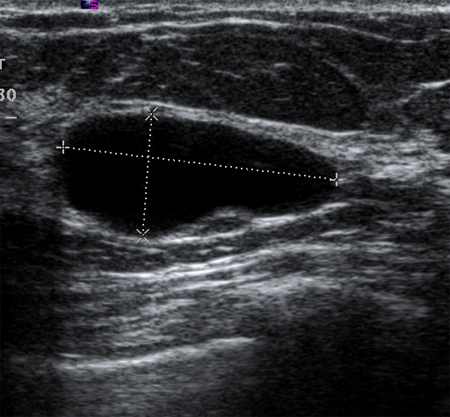
Suggested management for patients with "probably benign" masses on breast ultrasound includes:[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
Observation if clinical suspicion is low, with clinical examination and imaging with ultrasound or mammogram at 6, 12 and 24 months, to document stability.
Core needle biopsy to make a definitive diagnosis while leaving the lesion in situ. If the result is benign and concordant, a clinical breast exam every 6 to 12 months is recommended, with or without ultrasound or mammogram for 1 year to evaluate stability.
Surgical removal of the mass, particularly if the lesion is bothersome to the patient.
Ultrasonography of the axilla may also be performed to evaluate lymphadenopathy, and abnormal lymph nodes biopsied.[Figure caption and citation for the preceding image starts]: Diagnostic algorithm for breast ultrasoundCourtesy of Dr Anees Chagpar [Citation ends].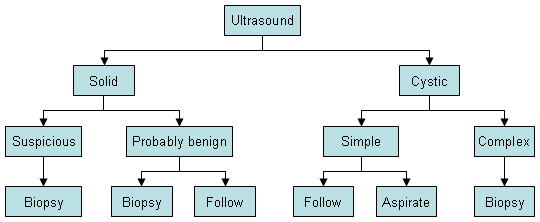
Magnetic resonance imaging (MRI) of the breast
National Comprehensive Cancer Network (NCCN) guidelines recommend considering MRI with and without contrast to investigate for inflammatory breast cancer if a patient has skin changes consistent with serious breast disease and is in BIRADS category 1-3, or is in BIRADS category 4-5 and has had a benign core needle biopsy.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
MRI can also be used to evaluate suspicious nipple discharge without a palpable mass if mammography and ultrasound findings are BIRADS 1-3.[19]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].
https://www.nccn.org/guidelines/category_2
The European Society of Medical Oncology recommends breast MRI for investigating suspected breast cancer if there are diagnostic uncertainties following breast ultrasound and mammography.[63]Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023 Dec 8 [Epub ahead of print].
https://www.annalsofoncology.org/article/S0923-7534(23)05104-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/38101773?tool=bestpractice.com
The US Preventive Services Task Force concludes that there is insufficient evidence to determine the balance of benefits and harms of supplemental screening for breast cancer with MRI (or with breast ultrasound) in women identified to have dense breasts on an otherwise negative screening mammogram.[65]US Preventive Services Task Force, Nicholson WK, Silverstein M, et al. Screening for breast cancer: US Preventive Services Task Force recommendation statement. JAMA. 2024 Jun 11;331(22):1918-10.
https://jamanetwork.com/journals/jama/fullarticle/2818283
http://www.ncbi.nlm.nih.gov/pubmed/38687503?tool=bestpractice.com
The American College of Radiology does not recommend MRI in the initial evaluation of a patient with a breast mass.[51]American College of Radiology. ACR appropriateness criteria: palpable breast masses. 2022 [internet publication].
https://acsearch.acr.org/docs/69495/Narrative
For patients undergoing MRI, diffusion-weighted imaging may be superior to contrast-enhanced MRI in differentiating benign from malignant lesions.[66]Chen X, Li WL, Zhang YL, et al. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010 Dec 29;10:693.
http://www.biomedcentral.com/1471-2407/10/693
http://www.ncbi.nlm.nih.gov/pubmed/21189150?tool=bestpractice.com
Biopsy is indicated for any suspicious lesion on MRI.
Breast aspiration and biopsy
A definitive diagnosis of breast carcinoma requires a breast biopsy. Three main types of biopsies are commonly performed.
Fine-needle aspiration (FNA): involves placing a 22- to 25-gauge needle into the breast mass and extracting cells. Increasing the number of passes increases the diagnostic odds ratio of FNA.[67]Akçil M, Karaağaoğlu E, Demirhan B. Diagnostic accuracy of fine-needle aspiration cytology of palpable breast masses: an SROC curve with fixed and random effects linear meta-regression models. Diagn Cytopathol. 2008 May;36(5):303-10.
http://www.ncbi.nlm.nih.gov/pubmed/18418880?tool=bestpractice.com
The cells can then be placed on a slide or made into a cell block. The advantages of FNA are that it is fast and easy to perform and it can be done in the doctor's office setting. The disadvantages are that it does not show histologic architecture, and it cannot help differentiate ductal carcinoma in situ from invasive malignancy. However, in the hands of an experienced cytopathologist, this technique may help distinguish benign from malignant lesions and is valuable for evaluating axillary lymph nodes.[67]Akçil M, Karaağaoğlu E, Demirhan B. Diagnostic accuracy of fine-needle aspiration cytology of palpable breast masses: an SROC curve with fixed and random effects linear meta-regression models. Diagn Cytopathol. 2008 May;36(5):303-10.
http://www.ncbi.nlm.nih.gov/pubmed/18418880?tool=bestpractice.com
[68]Yu YH, Wei W, Liu JL. Diagnostic value of fine-needle aspiration biopsy for breast mass: a systematic review and meta-analysis. BMC Cancer. 2012 Jan 25;12:41.
http://www.biomedcentral.com/1471-2407/12/41
http://www.ncbi.nlm.nih.gov/pubmed/22277164?tool=bestpractice.com
Core-needle biopsy: using an 8- to 14-gauge needle, provides a larger tissue sample than FNA. It can be performed by palpation, under stereotactic control, or by ultrasound guidance. This technique can be done in the doctor's office, is relatively fast and easy to perform, and allows for a histologic diagnosis. In the event of a malignant diagnosis, hormone receptor studies may be conducted on needle biopsy specimens. A variety of devices can be used to obtain these specimens, some using vacuum assistance, others radiofrequency energy.[69]Yu YH, Liang C, Yuan XZ. Diagnostic value of vacuum-assisted breast biopsy for breast carcinoma: a meta-analysis and systematic review. Breast Cancer Res Treat. 2010 Apr;120(2):469-79.
http://www.ncbi.nlm.nih.gov/pubmed/20130983?tool=bestpractice.com
[70]National Institute for Health and Care Excellence. Image-guided radiofrequency excision biopsy of breast lesions. Jul 2009 [internet publication].
http://www.nice.org.uk/guidance/IPG308
In general, core needle biopsy is the method of choice for histologic diagnosis of breast masses.[71]Hahn M, Krainick-Strobel U, Toellner T, et al. Interdisciplinary consensus recommendations for the use of vacuum-assisted breast biopsy under sonographic guidance: first update 2012. Ultraschall Med. 2012 Aug;33(4):366-71.
http://www.ncbi.nlm.nih.gov/pubmed/22723042?tool=bestpractice.com
Excisional biopsy: entails removing the entire breast mass for an accurate histologic diagnosis. This invasive technique, in the case of a benign asymptomatic mass, may be unnecessary; and in the case of a malignant mass, it may not obviate the need for a second procedure to treat the cancer once a diagnosis is made. Needle biopsy findings of atypical hyperplasia or radial scars require excisional biopsy to rule out concomitant malignancy.[Figure caption and citation for the preceding image starts]: Breast biopsy techniques (FNA; fine needle aspiration)Courtesy of Dr Anees Chagpar [Citation ends].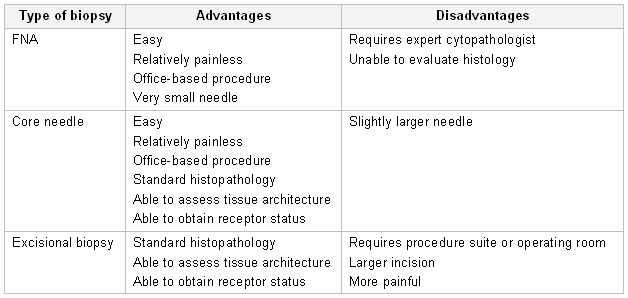
Painful cysts
May be aspirated under ultrasound guidance. Aspirated cyst fluid should not be sent for cytology, because, with the exception of bloody cystic fluid, malignant cells are generally not identified.[72]Hindle WH, Arias RD, Florentine B, et al. Lack of utility in clinical practice of cytologic examination of nonbloody cyst fluid from palpable breast cysts. Am J Obstet Gynecol. 2000 Jun;182(6):1300-5.
http://www.ncbi.nlm.nih.gov/pubmed/10871442?tool=bestpractice.com
Cysts that recur or do not completely resolve with aspiration should be biopsied to rule out malignancy. Similarly, biopsy should be considered in complex cysts or those with solid elements. Sonographic characteristics may classify a solid mass as either "probably benign" or "suspicious." Masses that are smooth, oval, lobulated, with clearly defined margins, and that are wider than they are tall, are often benign (e.g., fibroadenoma). If a mass is irregular, heterogeneous, has poorly defined or spiculated margins, and is taller than it is wide, it is considered "suspicious" for malignancy, and biopsy should be undertaken.[Figure caption and citation for the preceding image starts]: Ultrasonographic image of a complex cystCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Ultrasonographic image of a fibroadenomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ultrasonographic image of a fibroadenomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Ultrasonographic image of an invasive carcinomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ultrasonographic image of an invasive carcinomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends].

 [Figure caption and citation for the preceding image starts]: Obvious mass with skin involvement on left breastCourtesy of Dr Anees Chagpar [Citation ends].
[Figure caption and citation for the preceding image starts]: Obvious mass with skin involvement on left breastCourtesy of Dr Anees Chagpar [Citation ends].


 [Figure caption and citation for the preceding image starts]: Magnification view demonstrating irregular spiculated mass with associated calcificationsCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Magnification view demonstrating irregular spiculated mass with associated calcificationsCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Screening mammogram demonstrating breast massCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Screening mammogram demonstrating breast massCourtesy of Dr Nancy Pile, University of Louisville; used with permission [Citation ends].



 [Figure caption and citation for the preceding image starts]: Ultrasonographic image of a fibroadenomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ultrasonographic image of a fibroadenomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Ultrasonographic image of an invasive carcinomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ultrasonographic image of an invasive carcinomaCourtesy of Dr Lane Roland, University of Louisville; used with permission [Citation ends].