Etiology
In traumatic spinal cord injury (SCI), the initial injury is usually due to compression (from a space-occupying lesion, such as a hematoma) or physical trauma in the form of distraction, shearing, or laceration. In children, the spinal cord is particularly vulnerable because it does not tolerate any stretch. Injury can occur or be exacerbated even in the absence of radiologic abnormalities (a phenomenon known as SCI without radiologic abnormality [SCIWORA]).[3]
Nontraumatic SCI can be due to compressive (degenerative, tumors) or noncompressive (infectious, systemic inflammatory disorder, acquired demyelinating disorder, genetic, neurodegenerative, metabolic) causes. In older patients, recovery from SCI is diminished due to compromise of the vascular supply by degenerative changes in the cervical spine and atherosclerotic changes in the arteries supplying the cord. Older patients also have a higher incidence of metastasis of tumors to the spinal column, and of some primary tumors such as myeloma.
A narrow spinal canal (a developmental abnormality) increases the risk and severity of injury following trauma or a compressive lesion.[4]
Depending on the direction and magnitude of the forces causing SCI, some neurons and tracts can be preferentially involved. The amount and location of the intact neural tissue determines the residual function. Mechanical compression of the cord, which occurs over a period, allows for gradual compensatory adjustments that preserve function; compressive lesions are tolerated better than sudden mechanical distortion of the cord such as stretch, laceration, or shear. Vascular injuries (which can be primary or secondary to a mechanical injury) are poorly tolerated and have a poorer prognosis for recovery.
Following the initial injury, secondary spinal cord damage results from ischemia and/or inflammation.
Pathophysiology
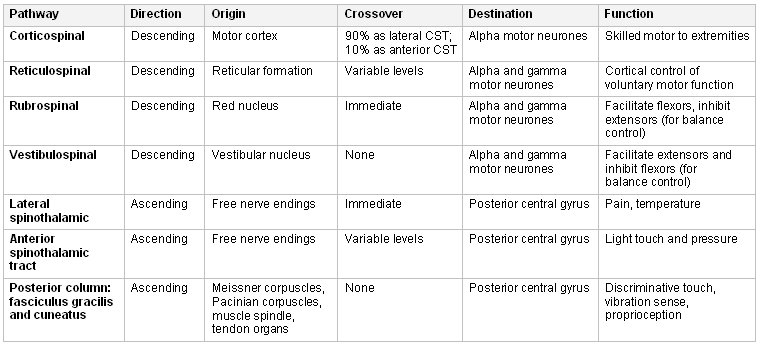
The disability produced by spinal cord injury (SCI) depends on the level of the injury and the tracts involved. The essential tracts for movement and sensation are:[5][6]
Reticulospinal, vestibulospinal, and rubrospinal tracts for integration of control of voluntary movement and balance
Corticospinal tracts for voluntary motor control and coordinated movements
Dorsal column and spinothalamic tracts for proprioception, fine touch, and nociceptive function.
Involvement of motor tracts causes an upper motor neuron lesion. Cervical lesions produce tetraplegia/tetraparesis, whereas thoracic and lower lesions produce paraplegia/paraparesis. Injuries of the lumbar spine cause an injury to the nerve roots or the cauda equina. Typically the involved limbs present with spasticity, hypertonia, hyperreflexia, a feeling of heaviness, stiffness, and an extensor plantar response in an upper motor lesion. When the injury involves the nerve roots, the affected limbs are flaccid and hypotonic (lower motor neuron type injury). Sensory changes include paresthesias, hyperesthesia, and proprioceptive changes commensurate with the level of the lesion.
Following the initial mechanical offense, secondary injuries such as ischemia may occur due to damage or occlusion of the main arteries or perfusing vessels, or a compromised microcirculation. Venous congestion secondary to arterial injury or systemic hypotension produced by autonomic dysfunction can also cause ischemia. The gray matter is especially vulnerable to ischemic injury because it is metabolically active and highly vascular.[7][8] Ischemic injury, if it occurs, is poorly tolerated by the spinal cord and is associated with a worse prognosis for recovery.
The initial injury can also be exacerbated by secondary inflammation. An inflammatory response is triggered by the tissue damage and the release of cytokines. Disruption of the blood-brain barrier may trigger a systemic inflammatory response.
Compensatory adjustments in the period after the initial injury allow function to be maintained in available uninjured neural tissue. These mechanisms tend to be most effective in slowly progressing injuries produced by compression, and least effective in ischemic injury. The final disability produced therefore depends on the level of the lesion, the amount of neural tissue spared, the extent of secondary injury from ischemia or inflammation, and the effectiveness of the compensatory responses that preserve function.
Chronic SCI can lead to a range of complications including (but not limited to) the following:
Disturbance in bladder function: micturition is a complex process under reflex and voluntary control. It involves the autonomic (parasympathetic and sympathetic) system, and the cortical centers that control the detrusor, internal, and external sphincters to maintain continence. Disturbance most commonly results in inappropriate bladder and sphincter activity, leading to either hyper-reflexic neurogenic lower urinary tract dysfunction in the case of an upper motor neuron lesion; or a flaccid, atonic bladder in the case of a lower motor neuron lesion.
Disturbance in bowel function: defecation and fecal continence also require complex coordination of the autonomic nervous system, peristalsis, spinal reflexes, and volitional input. Thus, SCI may lead to neurogenic bowel, characterized by insensate, poorly controlled bowel evacuation.
Respiratory dysfunction: patients with cervical spine injuries can develop paralysis of ventilatory muscles, leading to a decreased breathing and coughing capability. Disruption of autonomic input can lead to bronchoconstriction and excess secretions. Reduced mobility can produce a ventilation/perfusion mismatch that can exacerbate hypoxia if there is an associated illness.
Sexual dysfunction: disruption of the autonomic pathways can reduce the ability for erection and ejaculation in men and vaginal secretions in women. Altered sensation, impeding enjoyment of intercourse, can affect both sexes.[9] In several studies, around 60% of respondents had post-injury amenorrhea, with the average time until menses resumption being 5 months. Although fertility is not generally affected in women following return of menses, a drop in pregnancy rates has been reported, especially in patients with tetraplegia, suggesting additional contributing factors.[10][11][12]
Joint contractures: a loss of spinal cord innervation leads to muscle wasting and fibrous replacement. This, combined with spasticity, can lead to flexion contractures as these structures shorten across joints such as the elbow, hip, knee, or ankle.
Neuropathic pain: occurs due to a heightened sensitivity of the receptors or inhibition of descending pathways.
Autonomic dysreflexia: can occur in patients with a lesion affecting T6 or higher level due to disruption of the sympathetic chain and balance with the parasympathetic nervous system. It is caused by an excessive autonomic response to stimuli below the level of the lesion, such as a fecal impaction or blocked catheter. This produces sympathetic overactivity below, and parasympathetic overactivity above, the level of injury. This can present as a sudden uncontrolled rise in blood pressure and a range of other symptoms, including pounding headache, sweating or shivering, anxiety, chest tightness, blurred vision, nasal congestion, blotchy skin rash or blushing above the lesion level, and cold with goosebumps (cutis anserina) below the level of the injury.[Figure caption and citation for the preceding image starts]: Neuroanatomy and functions of the spinal tracts. CST, corticospinal tractsFrom personal collection of Dr Jwalant S. Mehta [Citation ends].
Classification
American Spinal Injury Association (ASIA) International Standards for Neurological Classification of Spinal Cord Injury
The ASIA Impairment Scale classifies the injury as A, B, C, D, or E according to the degree of residual neurologic function: SCIRE: international standards for neurological classification of spinal cord injury Opens in new window
A: no motor or sensory function
B: preserved sensory function including S4-S5 (sacral sparing) but no motor function
C: preserved sensory function including S4-S5 (sacral sparing); muscle flicker or full range of motion (ROM) with gravity eliminated
D: preserved sensory function including S4-S5 (sacral sparing); full ROM against gravity or added resistance
E: preserved sensory function including S4-S5 (sacral sparing); full ROM with normal strength; spasticity or long tract signs.
Lesions can also be classified as complete or incomplete:
Complete: any type A injury
Incomplete: any type B, C, D, or E injury.
Spinal cord syndromes
The pattern of weakness and loss of sensation due to the disruption of spinal tracts can additionally be described by several classic syndromes. The clinical exam may reveal a characteristic spinal cord syndrome that should be recognized. Spinal cord syndromes include:
Brown-Sequard syndrome: produces root pain at the level of the lesion with loss of pain and temperature modalities below the lesion on the ipsilateral side, and over the lower extremity and the trunk on the contralateral side. The sensory level is 2 levels lower on the contralateral side. A spastic weakness is seen on the ipsilateral side with brisk reflexes. The posterior column may be involved on the ipsilateral side, producing loss of proprioception, and urgency may be noted.
Complete cord transection: a bilateral sensory level at the level of the lesion affecting all the modalities, which are either absent or severely affected. The upper margin may show a zone of hyperesthesia. Flaccid paraplegia or tetraplegia are seen. Urgency and incontinence occur due to a reflex neurogenic lower urinary tract dysfunction.
Posterior column compression: although no longer included as a formal syndrome, this produces loss of proprioception, with an associated spastic paraparesis and brisk reflexes. There may be radicular pain along the intercostal nerve. The sensory level to pain on the trunk is 2 levels below the level of the lesion. A lesion of T12 or above produces loss of abdominal reflexes. Urgency and incontinence are seen. Lesions above T5 may produce autonomic dysfunction such as orthostatic hypotension, sweating, and bradycardia with bladder or rectal stimulatory response of distension.
Central cord syndrome: caused by a lesion that affects the inner tracts in the cervical cord. Early in the pathology, the symptoms are predominantly sensory with a zone of pain and temperature loss 2 to 3 levels caudal to the lesion. If the lesion expands, motor involvement occurs producing bilateral spastic paraparesis of the lower extremities and asymmetric upper extremity paraparesis; neurologic impairment is more severe in the upper extremities.
Anterior cord syndrome: this is due to vascular injury involving the anterior vertebral artery. This may be due to a flexion injury involving the anterior two-thirds of the spinal cord, which injures the anterior vertebral artery but spares the paired posterior vertebral arteries. Pain, temperature, and motor function below the level of the lesion are impaired, but proprioception is intact.
Use of this content is subject to our disclaimer