Approach
Intra-abdominal hypertension should ideally be detected and treated before abdominal compartment syndrome (ACS) develops. A series of medical maneuvers exists to reduce intra-abdominal pressure (IAP), and these should always be employed regardless of whether the patient presents with intra-abdominal hypertension or ACS. These include:
Treatment of the underlying cause.
Supportive care with monitoring of IAP, analgesia, and correct body positioning.
Optimization of fluid balance. This involves avoidance of excessive fluid resuscitation, the use of colloids and hyperosmotic agents and 25% human albumin, and treatment with diuretics.
Evacuation of intraluminal contents. Small bowel contents can be evacuated using nasogastric (NG) gastroprokinetic agents and limitation of enteral feeding. Large bowel contents can be evacuated by a rectal tube, coloprokinetic agents, enemas, and, as a last resort, colonoscopic decompression.
Adherence to a defined algorithm of early surveillance and intervention has led to improved outcomes in patients with intra-abdominal hypertension/ACS.[35] The emphasis on the above strategies will depend on the underlying cause. For example, if the patient has received excessive fluid resuscitation, aggressive management of fluid balance will be pursued. However, if the underlying cause is ileus, evacuation of intraluminal contents will be pursued.
The definitive treatment of ACS, surgical decompression, should only be employed if and when medical therapy proves to be inadequate, either by paracentesis or by surgery. Some patients may require mechanical ventilation to overcome the effects of pressure transmission to the torso from the abdomen. Neuromuscular blockade is an extreme measure that can be used to reduce abdominal wall compliance. Patients with renal failure may require dialysis or hemofiltration to treat their renal failure and restore neutral fluid balance.
Treatment of underlying cause
A primary cause such as abdominal bleeding or trauma, packing, intra-abdominal infection/inflammation, ileus, pneumoperitoneum, or bowel ischemia should be managed; most of these conditions require surgical intervention, which may already have been performed prior to the development of ACS.
Secondary causes are almost always related to excessive fluid resuscitation and massive blood transfusions, which require optimization of fluid balance. Escharotomies may need to be performed in patients with thermal injuries.
Supportive care
All patients require supportive care with regular monitoring of IAP and oxygen saturations. Adequate analgesia and sedation should be provided. In addition to providing pain relief, this helps relax the abdominal musculature and improves abdominal compliance.
Although important in other critical care bundles, elevated head of bed may worsen abdominal compliance; therefore, lowering bed tilt to less than 30° should be considered in patients with suspected poor abdominal compliance. IAP may decrease if the patient is placed flat (supine position), or even placed into the reverse Trendelenburg position (patient flat, head up, feet down). Reverse Trendelenburg may specifically mitigate diaphragmatic displacement and improves lung compliance. It may also improve abdominal compliance. Some patients may not tolerate a reverse Trendelenburg position, due to a drop in mean arterial pressure or cardiac output.[10] Tight or constrictive clothing places pressure on the abdomen and should be removed. Any bandages that increase the tension on the abdominal cavity should also be removed where possible.
The need for organ support should be assessed in all patients. Some patients require mechanical ventilation to overcome the effects of pressure transmission from the abdomen to the thorax. Abdominal perfusion pressure should be maintained at or above 60 mmHg, which may require the use of vasopressors.
Optimization of fluid balance
Positive fluid balance, if present, should be corrected to return the patient to neutral fluid balance. Careful assessment of volume status and cardiac function is required.
Excessive fluid resuscitation should be avoided, and employing a restrictive fluid resuscitation strategy has clear benefits that outweigh aggressive fluid removal in the setting of elevated IAP.[24]Colloids and hypertonic crystalloids should be used rather than isotonic or hypotonic crystalloids. To exert intravascular osmotic pressure and return fluid to the intravascular compartment, 25% human albumin can be given, although its effectiveness has not been confirmed in clinical trials.
Diuretic therapy can be successfully employed in fluid-overloaded patients whose kidneys will respond to these agents. Care must be taken to monitor preload to avoid exacerbating shock in critically ill patients who are hypovolemic. However, excessive fluid loss is easier to correct than excessive fluid administration. It is better to start with a higher dose so that effective diuresis is achieved quickly. The major toxicity of diuretic agents, particularly if high doses are used, is ototoxicity. These agents should therefore be administered via infusion pump at a controlled rate.
Measurement of the fluid status with central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP) can be erroneously elevated because of the transmural effect of increased IAP on these measurements. The abdomino-thoracic pressure transmission is estimated to be around 50% of the IAP. Therefore, calculating CVP = CVP-(IAP/2) and the PAOP = PAOP-(IAP/2) gives better estimates of these pressures.[36]
Evacuation of intraluminal contents
Evacuation of intraluminal gastric contents using an NG tube reduces pressure exerted by accumulation of gastric contents. An NG tube is usually already present in ICU patients and should be strongly considered. NG decompression is more likely to decrease pressure when gastric distension is from fluid rather than air (more compressible).
Small bowel contents can be evacuated using an NG tube, gastroprokinetic agents, and limitation of enteral feeding. Gastroprokinetic agents (e.g., erythromycin) should also be considered, especially if ileus is the underlying cause. Limitation or discontinuation of enteral feeding may be considered if these measures are inadequate.
Colonic decompression may have a minor impact on IAP. As with NG decompression, removal of fluid or impacted stool is most likely to be of benefit. Coloprokinetic agents (e.g., neostigmine) and enemas should be considered if the response to tube placement is inadequate. Colonoscopic decompression may be considered as a final option.
Abdominal decompression
Paracentesis with percutaneous decompression has been described primarily in patients with burns. A peritoneal lavage or dialysis catheter is placed percutaneously into the peritoneal cavity and set to gravity drainage. If free fluid volumes are large, intra-abdominal pressure (IAP) is quickly lowered. Pathophysiologic processes that stimulate production of free peritoneal fluid are more amenable to this approach. Bedside ultrasound may facilitate identification and drainage of fluid collections.[37] Successful percutaneous decompression has been associated with fluid drainage of >1000 mL or a decrease in IAP of >9 mmHg in the first 4 hours post decompression.[38] If ACS persists after percutaneous drainage, then surgical treatment should be given immediately.[39]
A delay in the definitive treatment of ACS with surgical decompression often leads to a progressive increase in the risk of mortality.[24]
Open abdominal decompression is the definitive treatment of ACS but is reserved for patients in whom other interventions have failed.[10][40][41][42] Hypotension, oliguria, and elevated airway pressure, if present, resolve rapidly after the procedure. Although general anesthesia and the presence of a surgeon are required, the procedure can be safely performed at the bedside in the ICU.[39] This is an important advantage, because many patients with ACS are clinically unstable.
It is accomplished by performing a midline laparotomy incision. Care should be taken to avoid inadvertent injury to the bowel, which may bulge as soon as the peritoneal cavity is entered. The most restrictive abdominal layer is the fascial layer, and this should be opened generously in cephalad and caudad directions. A variety of temporary closure methods have been described, but in most cases both the fascia and the skin are left open.[43][44][45] A dressing must be applied to prevent dessication of the viscera. More recently there has been considerable enthusiasm for negative pressure/vacuum therapies, which have the added benefit of removing fluid that might otherwise reaccumulate and cause a recurrence of ACS. Additionally, the utilization of negative pressure therapy may reduce the transmission of inflammatory mediators to the bloodstream, potentially slowing the progression of multiorgan dysfunction.[24]
Patients undergoing decompressive laparotomy will later require permanent abdominal wound closure. Options range from delayed primary fascial closure and various flap/fascial release measures[46][47] to mobilization of skin flaps or split-thickness skin grafting. All of these measures are associated with a ventral hernia that requires separate, late management.[48] The utilization of negative pressure wound therapy devices has increased in recent years and reports suggest that these devices are associated with increased fascial closure rates and decreased length of hospital stays compared with traditional temporary abdominal closures.[49][50] However, there is potential for wound vacuum devices to cause tertiary ACS if the vacuum pressure is too high; therefore, ongoing monitoring should continue and medical management of intra-abdominal hypertension/ACS should persist after the abdomen is opened as this will facilitate the earlier closure of the fascia.[Figure caption and citation for the preceding image starts]: "Bogota bag" temporary abdominal closure after surgical decompression of abdominal compartment syndromeFrom the personal collection of Michaela A. West, MD, PhD [Citation ends].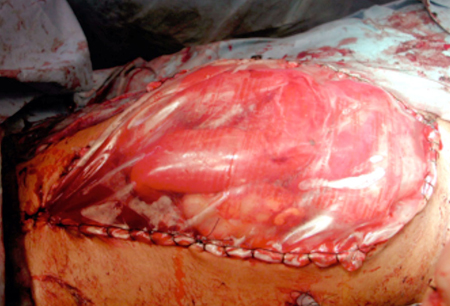
Additional treatments
Neuromuscular blockade
Complete neuromuscular blockade can be given as a last resort to patients to decrease the compliance of the abdominal wall. Patients will require airway control and mechanical ventilation.
Sedative and neuromuscular blocking agents may decrease BP and/or cardiac output, which may be problematic in hypotensive patients who remain in shock.
It is important to monitor the adequacy of neuromuscular blockade, especially if IAP increases while patients are receiving this treatment.[51][52]
Dialysis
This may effectively mobilize excess fluid and is particularly useful in the setting of acute or chronic renal failure, when diuretic therapies are not feasible. Dialysis takes hours to days to be effective, but under some circumstances net removal of even 1 to 2 liters of excess fluid can significantly lower IAP.
Disadvantages include the need for higher flow intravascular catheters and the complications that may arise from their insertion, such as infection. Also, dialysis requires trained personnel and specialized equipment, which, while widely available, is not immediately accessible in all ICUs.
Use of this content is subject to our disclaimer