Details
Thyroid function tests (TFTs) are the most commonly used endocrine test. American Thyroid Association: thyroid function tests Opens in new window Thyroid hormones thyroxine (T4) and triiodothyronine (T3) are produced, stored, and secreted by the thyroid gland. These hormones, particularly T3, play a major role in multiple biologic and metabolic processes. They act by binding to thyroid receptors that are distributed in almost every organ. Typically, this process regulates gene transcription and the subsequent production of various proteins that are involved in development, growth, and cellular metabolism.[1] This topic discusses indications for TFTs and the interpretation of results, in particular serum thyrotropin (TSH) levels and associated free T3 and free T4 levels.
Thyroid hormone production is regulated by the hypothalamus and pituitary gland. Hypothalamic thyrotropin-releasing hormone (TRH) stimulates pituitary TSH synthesis and secretion. In turn, TSH stimulates production and release of T4 and T3 from the thyroid gland. Once released, T4 and T3 exert a negative feedback mechanism on the production of TRH and TSH.[1][Figure caption and citation for the preceding image starts]: Hypothalamic-pituitary-thyroid axisFrom the collection of Dr Sheikh-Ali [Citation ends].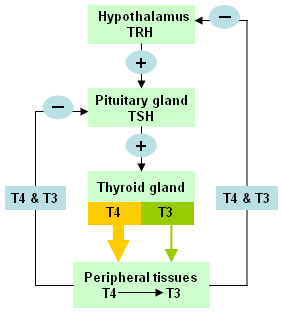
The protein thyroglobulin (Tg) is produced and used by the thyroid gland to produce T4 and T3. T3 is the biologically active form of thyroid hormone whereas T4 is considered a prohormone to T3. The thyroid gland produces 100% of circulating T4 but only 20% of circulating T3. The remaining 80% of T3 is produced by the conversion of T4 to T3 in the peripheral tissues. Acute illnesses, as well as certain drugs, may inhibit the process of converting T4 to T3 and, therefore, affect their serum levels.
T3 and T4 circulate in peripheral blood bound to proteins (thyroxine-binding globulin [TBG], transthyretin [previously prealbumin], and albumin). Over 99% of T4 and T3 are protein-bound. Only the unbound or "free" portion, free T3 (FT3) and free T4 (FT4), are active. Therefore, any changes in the quantity or quality of thyroid-binding proteins will produce changes in circulating thyroid hormone levels.[2]
According to population studies in the US and the UK, the prevalence of overt hypothyroidism varies from 0.1% to 2%, and of subclinical hypothyroidism from 4% to 10% of adults. Incidence is higher in women than in men.[3][4] The overall prevalence of thyroid disease varies widely based on population. In the US, the prevalence of hyperthyroidism is approximately 1.2% (0.5% overt and 0.7% subclinical); the most common causes include Graves disease, toxic multinodular goiter, toxic adenoma, and excess thyroid hormone ingestion.[3][5]
In the US, the American Thyroid Association guidance on thyroid dysfunction suggested that all adults should have serum TSH concentration measured at 35 years of age and every 5 years thereafter.[6] The American Association of Clinical Endocrinologists is also in favor of screening in "older people" (age not specified), especially women.[7] However, the US Preventive Services Task Force found that evidence was insufficient to recommend for or against routine screening for thyroid disease in adults.[8] In the UK, Canada, and Australasia, the healthy adult population is not routinely screened for thyroid disease.[9][10][11][12]
Screening may be appropriate in people at higher risk of developing thyroid dysfunction. For example, people with Down syndrome are more frequently affected by both hypothyroidism and hyperthyroidism, and the symptoms may be masked by their diagnosis. The Down syndrome Medical Interest Group (DSMIG) therefore recommends screening all infants with Down syndrome at ages 4-6 months, at ages 12 months and annually thereafter.[13]
Screening and further surveillance should also be considered in patients:[14][15][16][17]
With a goiter
Who have had surgery or radiation therapy affecting the thyroid gland
Who have pituitary or hypothalamic disease, surgery, or irradiation
With diabetes mellitus type 1
With Addison disease
With first-degree relative with autoimmune thyroid disease
With vitiligo
With pernicious anemia
With leukotrichia (prematurely gray hair)
With psychiatric disorders
With Turner syndrome
Receiving certain medications and iodine-containing compounds (e.g., amiodarone, radiocontrast agents, expectorants containing potassium iodide, kelp, interferon alfa, and tyrosine-kinase inhibitors, most notably sunitinib).
TSH measurement is also recommended before starting certain treatments that can affect thyroid function. This includes immune reconstitution therapy (e.g., alemtuzumab treatment for multiple sclerosis, antiretroviral therapy for HIV infected patients, allogeneic bone marrow transplantation, or hematopoietic stem cell transplantation), and treatment with immune checkpoint inhibitors.[18][19][20]
In patients with known history of hypothyroidism who are trying to conceive, thyroxine levels should be increased to achieve serum TSH values to <2.5 mIU/mL. This increase will reduce the risk of TSH elevation during the first trimester.[21]
In pregnancy, estrogen levels increase and thyroid-binding globulin concentrations rise, which leads to an increase in T4 and T3.[22] In the first trimester, serum TSH also falls due to the effect of human chorionic gonadotropin (hCG), which may be associated with a slight and transient increase in FT4. These changes are small, and in most pregnant women FT4 concentrations remain within the normal range for nonpregnant women.[23] In the second and third trimesters, FT4 and FT3 decrease, sometimes below the nonpregnant women's reference level. There is insufficient evidence to recommend for or against screening for thyroid dysfunction in pregnant women.[22]
In a randomized controlled trial of 4562 women in the first trimester, universal screening compared with case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy did not result in a decrease in adverse outcomes.[24] The American College of Obstetricians and Gynecologists (ACOG) recommends testing for thyroid dysfunction only in pregnant women with:[25]
History of hyperthyroid or hypothyroid disease, postpartum thyroiditis, or thyroid lobectomy
Family history of thyroid disease
Symptoms or clinical signs suggestive of thyroid under-function or over-function
Type 1 diabetes mellitus
TSH levels should be monitored closely in pregnant women with hypothyroidism because thyroxine replacement often needs to be increased between 20% and 30% during the first trimester.[22] Thyroxine dosage should be titrated to maintain serum TSH concentrations of 0.1 to 2.5 mIU/mL in the first trimester, 0.2 to 3 mIU/mL in the second trimester, and 0.3 to 3 mIU/mL in the third trimester.[21][24] Monitoring during pregnancy should consist of measuring TSH every 4 weeks for up to 20 weeks' gestation, and then at least once at 26 and 32 weeks of gestation. After delivery, thyroxine should be reduced to prepregnancy dose and TSH rechecked at 6 weeks to further adjust thyroxine if needed.[21]
Congenital hypothyroidism (CH) is a treatable cause of cognitive impairment and growth failure that affects about one in 3500-4000 newborns.[3][26] Most developed countries routinely test for CH by measuring serum TSH via a heel-prick blood sample in the first few days after birth. If TSH is elevated, neonates should be referred to a specialist, and testing of serum FT4 and TSH should be carried out within a short turnaround time to allow for timely treatment.[26][27] Monitoring of TSH and FT4 in infants with primary CH should continue at regular intervals, and at ages 3 years their status should be reevaluated. The reference ranges used to interpret test results must be age-specific.[26][27]
TSH assay
A serum TSH assay is the test of choice to screen for thyroid function disorders in the absence of hypothalamic or a pituitary pathology.[6][28][29][30] In most reference laboratories, the normal range for TSH is 0.45 to 4.5 mIU/L.[31] The reference interval for TSH is a function of age, and age-specific reference values are particularly important in neonates and older adults.[27][32] TSH is sensitive to any change in the plasma concentration of thyroid hormones.[33] TSH may require an average of 6-8 weeks to adjust to changes in thyroid hormone levels. Therefore, it is recommended to check TSH levels 6-8 weeks after thyroxine adjustment or any antithyroid drug treatment.
Suppressed or elevated TSH confirms presence of thyroid dysfunction but not its cause. A subnormal TSH level should trigger the measurement of FT4 and FT3.[34] Rarely, TSH alterations can be caused by assay interference with heterophilic antibodies (antibodies to mouse IgG or other human anti-mouse monoclonal antibodies [HAMAs]). This may result in falsely low or high TSH results, although usually it causes elevated serum TSH. Repeat TSH testing using different commercial assays can neutralize the effect of heterophilic antibodies.[31] Biotin immunoassay interference can cause falsely decreased TSH.[2]
Free T4 (FT4) and free T3 (FT3) assays
FT4 assay is the test of choice to evaluate an abnormal TSH level. It is used in preference to a total T4 assay. FT3 should be measured to identify cases of T3-thyrotoxicosis. Typical normal range for FT4 is 0.9 to 2.3 nanograms/dL (12 to 30 picomol/L) and for FT3 is 230 to 420 picograms/dL (2 to 7 picomol/L). Biotin immunoassay interference can cause falsely increased FT4.[2] FT3 measurements are susceptible to interference by free fatty acids and drugs present in the circulation. Some laboratories prefer to run total T3 assays for this reason.[35]
Total T4 and total T3 assays
Previously, before improved FT4 and FT3 assays, total T4 and total T3 assays were ordered to evaluate an abnormal TSH assay. However, total T4 and total T3 levels can be affected by changes in the levels of circulating thyroid hormone-binding protein levels. They measure both free and protein-bound hormones. Normal range for total T4 is 5.5 to 12.5 microgram/dL (206 to 309 nanomol/L) and normal range for total T3 is 60 to 180 nanograms/dL (0.92 to 2.76 nanomol/L).
Conditions associated with elevated total T4 and total T3 levels secondary to increased thyroxine-binding globulin (TBG) levels include pregnancy, estrogen use, liver diseases (e.g., hepatitis), drug use (e.g., tamoxifen or methadone), or rarely, hereditary TBG excess.[36][37] Other rare conditions resulting in elevated total T4 and total T3 levels are increased albumin or transthyretin binding.
Conditions associated with decreased total T4 and total T3 levels secondary to decreased TBG levels include androgen excess, glucocorticoid excess, nephrotic syndrome, hereditary TBG deficiency, and drug use (e.g., niacin or danazol).[37][38][39]
Illness, starvation, and poor nutrition may also decrease total T4 and total T3 levels by decreasing albumin and transthyretin levels and possibly interfering with the binding capacity of the carrier proteins.
Thyroid autoantibodies
TSH-receptor antibodies (TRAb) are not routine tests but may be of use in selected cases where diagnosis is equivocal. The results are useful in identifying thyroid disease etiology (e.g., Graves disease).[34] TRAb can be either stimulatory or blocking to the TSH receptor. Thyroid-stimulating immunoglobulin (TSI) is an example of a stimulatory TRAb and is usually elevated in Graves disease.
Thyroid peroxidase antibodies (TPOAb) are also helpful in identifying thyroid disease etiology. TPOAb are usually present in Hashimoto disease and other autoimmune thyroid diseases.
Thyroglobulin (Tg) antibody test is used primarily to help diagnose autoimmune conditions involving the thyroid gland.
Because TRAbs freely cross the placenta and stimulate the fetal thyroid gland, TRAb should be measured in early pregnancy, and if elevated repeated at 18-22 weeks of gestational age in a mother with current or a history of Graves disease, previous neonate with Graves disease, or previously elevated TRAb. If still elevated, TRab testing should be repeated at 30-34 weeks to evaluate the need for perinatal monitoring.[22]
Tg assay
Usually ordered for surveillance in patients with differentiated thyroid cancer when the patient does not have Tg autoantibodies in the serum.
In addition, it may be ordered when investigating the underlying cause of hyperthyroidism. Tg is usually elevated in primary hyperthyroidism and thyroiditis but not in factitious thyrotoxicosis (excessive use of thyroid hormone medication causing thyrotoxicosis).
An increase in serum Tg occurs in 33% to 88% of patients who undergo thyroid fine needle biopsy (FNB). Serum Tg concentrations typically return to baseline about 2-3 weeks after FNB. The degree of increase in serum Tg after FNB is highly variable (ranging from 35% to 341%) and not a predictor of whether the biopsied nodule is benign or malignant.[40]
Radioactive iodine uptake (RAIU) and scan
Usually ordered in the setting of thyrotoxicosis to help identify the underlying etiology. It measures the amount of radioactive iodine (usually I-123) that is taken up by the thyroid gland. High uptake may indicate hyperthyroidism. The increased uptake may be diffuse and homogeneous as seen in Graves disease, or take on the appearance of hot nodules, as seen in multinodular toxic goiter. Low uptake may indicate thyroiditis or factitious thyrotoxicosis in the appropriate clinical setting.
RAIU cannot be performed in certain patients (e.g., pregnant or nursing women or iodine-contaminated patients); in such cases, serum TRAb measurement is helpful in identifying Graves disease.
TRH stimulation test
Used to evaluate TSH response to TRH stimulation in the setting of central hypothyroidism. It may also help differentiate TSH secretory tumor from resistance to thyroid hormone syndrome (RTH). In RTH, the TSH response is normal. TRH stimulation test is not a specific test and is not commonly available in the US.
Calcitonin
Calcitonin is usually a marker of medullary thyroid cancer.[41] However, calcitonin levels may also be increased, although infrequently, in other clinical conditions such as C-cell hyperplasia, autoimmune thyroiditis, prostate cancer, pulmonary and pancreatic neuroendocrine tumors, renal failure, and hypergastrinemia (use of proton-pump inhibitors).[41][42]
A single, unstimulated calcitonin measurement can be used in the initial workup of thyroid nodules.[42] However, this practice is not done routinely in the US, and in the UK it is only recommended when medullary carcinoma is suspected.[43]
Suggestive of hyperthyroidism. The most common causes include Graves disease, toxic multinodular goiter, toxic adenoma, and thyroiditis.[44] Radioactive iodine uptake (RAIU) helps to differentiate between these conditions. In cases of Graves disease, RAIU is diffusely increased. For toxic nodules: uptake is increased in the area of a single nodule, or in multiple areas in cases of toxic multinodular goiter; the remaining thyroid tissue uptake is suppressed. Furthermore, in Graves disease, thyroid-stimulating immunoglobulins (TSI) are present in about 90% of patients, though usually not required for diagnosis.
In subacute or granulomatous thyroiditis, RAIU is low. Patients with subacute thyroiditis have elevated thyroid hormone levels initially, secondary to excessive release of stored T4 and T3 from the thyroid gland. Later, thyroid hormone levels decrease below normal, before returning to normal when inflammation subsides.
Other causes include factitious thyrotoxicosis (caused by excessive use of thyroid hormone medication), in which case thyroglobulin levels and RAIU are low, or iodine-induced hyperthyroidism (e.g., following use of amiodarone or exposure to radiocontrast agents), where RAIU is also low. [Figure caption and citation for the preceding image starts]: Differentiating causes of low TSH and high free T4 (FT4) and/or free T3 (FT3)From the collection of Dr Sheikh-Ali [Citation ends].

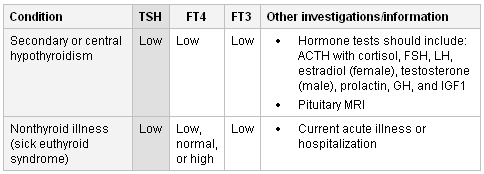
Suggests secondary (central) hypothyroidism, which is associated with pituitary or hypothalamic dysfunction. TSH can be low, normal, or slightly elevated. Evaluation for deficiencies in other pituitary hormones should be obtained before imaging (i.e., pituitary MRI). Hormone tests should include: ACTH with cortisol, FSH, LH, estradiol (female), testosterone (male), prolactin, GH, and insulin-like growth factor 1. For this condition, thyroid replacement therapy is monitored by checking the levels of FT4 and FT3.[45]
Other causes of these results include nonthyroid illness (sick euthyroid syndrome) where abnormalities in thyroid tests secondary to acute systemic illness are observed with no true thyroid dysfunction. TSH can be normal or low followed by rebound elevation during recovery from acute illness.[31] FT4 can be normal, low, or high. FT3 is usually low secondary to decreased conversion of T4 to T3. Thyroid hormone replacement is not recommended in this condition.[46][47]
In the second and third trimesters of pregnancy, FT4 and FT3 decrease, sometimes below the nonpregnant woman's reference level. [Figure caption and citation for the preceding image starts]: Differentiating causes of low TSH and low free T4 (FT4) and/or free T3 (FT3)From the collection of Dr Sheikh-Ali [Citation ends].
In the absence of nonthyroidal illness or relevant drug therapy, these results suggest subclinical (or mild) hyperthyroidism. In this case radioactive iodine uptake (RAIU) can be slightly elevated or normal.
In nonthyroid illness (sick euthyroid syndrome), TSH can be normal or low followed by rebound elevation during recovery from acute illness.[31] FT4 can be normal, low, or high. FT3 is usually low secondary to decreased conversion of T4 to T3.
The following drugs may cause these results: dopamine, dopaminergic agonists, glucocorticoids, cytokines, or octreotide, because they inhibit pituitary TSH secretion. Similar results may also occur following exposure to radiocontrast agents.[48][49]
Recent treatment of hyperthyroidism with antithyroid medication may also cause these results. It may take up to 6-8 weeks for TSH to adjust after initiating therapy.[50]
In the first trimester of pregnancy, serum TSH falls due to the effect of human chorionic gonadotrophin. This may be associated with a slight and transient increase in FT4.[23][Figure caption and citation for the preceding image starts]: Differentiating causes of low TSH and normal free T4 (FT4) and/or free T3 (FT3)From the collection of Dr Sheikh-Ali [Citation ends].

African-American people may have slightly lower TSH reference ranges for normal FT4 and FT3 compared with white people.[51]
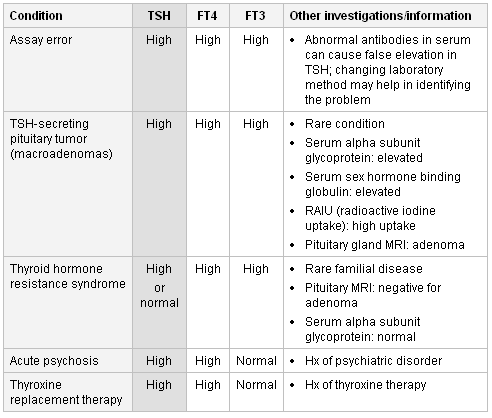
If this result is found then assay artifact/laboratory error should be considered first. Changing laboratory method may help in identifying the problem.[52]
If assay results are correct, the major diagnoses are a TSH-secreting pituitary tumor (TSH-oma) or a syndrome of resistance to thyroid hormone. The finding of an elevated serum sex hormone-binding globulin (SHBG) and circulating free alpha subunit may support the diagnosis of TSH-oma, as may the finding of hyper- or hyposecretion of other pituitary hormones.[53] Pituitary imaging (MRI) usually confirms the diagnosis but should not be undertaken until the appropriate biochemical confirmation has been made. Radioactive iodine uptake (RAIU) shows diffusely increased uptake.
Thyroid hormone resistance syndrome can be confirmed by positive family history, absence of adenoma on pituitary MRI, and normal levels of serum alpha subunit glycoprotein. By contrast, with a TSH-oma where TSH production is autonomous, T4 or T3 administration eventually suppresses the high TSH in thyroid hormone resistance syndrome.[54][55][56]
Thyroxine replacement therapy (for possible hypothyroidism) taken within a few hours of TFT can raise FT4 levels. However, FT3 is almost always normal in these situations.[57]
Abnormal thyroid function has been found in patients with acute psychiatric disorders.[58][Figure caption and citation for the preceding image starts]: Differentiating causes of high TSH and high free T4 (FT4) and/or free T3 (FT3)From the collection of Dr Sheikh-Ali [Citation ends].
Suggests primary hypothyroidism. Underproduction of the thyroid hormones (T4 and T3) may occur with autoimmune thyroiditis (Hashimoto disease), which is the most common cause of primary hypothyroidism. More than 90% of patients with Hashimoto thyroiditis have positive TPOAb.
Other causes include thyroidectomy or radioactive iodine treatment of the thyroid without adequate thyroid hormone replacement.[Figure caption and citation for the preceding image starts]: Differentiating causes of high TSH and low free T4 (FT4) and/or free T3 (FT3)From the collection of Dr Sheikh-Ali [Citation ends].

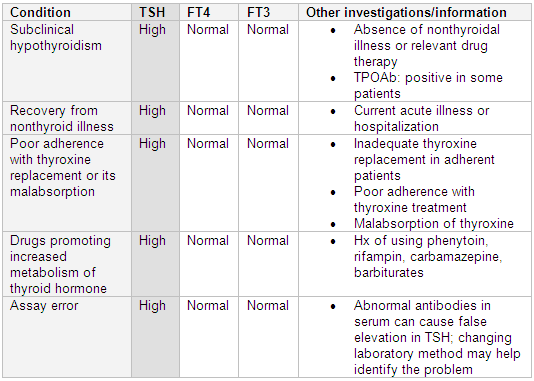
Subclinical (or mild) hypothyroidism occurs when TSH is above reference range with a normal FT4 and FT3. The risk of progression to overt hypothyroidism is 2% to 5% per year.[59] The risk is higher in patients with positive TPOAb.[60] The decision to treat these patients is controversial. Generally, thyroxine replacement is not recommended when TSH is below 10 mIU/L.[26] TSH and FT4 should be repeated at 6- to 12-month intervals to monitor for improvement or worsening in thyroid status in untreated patients.[59]
Differentials include the recovery from nonthyroid illness (sick euthyroid syndrome). TSH can be normal or low followed by rebound elevation during recovery from acute illness.[31]
Other differentials include poor adherence to thyroxine replacement therapy or its malabsorption: for example, in celiac sprue, or as a result of interference from other co-administered medications, such as calcium carbonate, ferrous sulfate, and cholestyramine.
Concomitant medication that promotes increased metabolism of thyroid hormone (e.g., rifampin, phenytoin, carbamazepine, barbiturates) can also cause these results.[31]
In addition, problems with assay procedures (e.g., interference of abnormal antibodies in serum) can cause false elevation in TSH. Changing the laboratory method may help identify the problem.[52][Figure caption and citation for the preceding image starts]: Differentiating causes of high TSH and normal free T4 (FT4) and/or free T3 (FT3)From the collection of Dr Sheikh-Ali [Citation ends].
These results may occur following secondary (central) hypothyroidism, which is associated with pituitary or hypothalamic dysfunction. TSH can be low, normal, or slightly elevated. Evaluation for deficiencies in other pituitary hormones should be obtained before imaging (i.e., pituitary MRI). Hormone tests should include: ACTH with cortisol, FSH, LH, estradiol (female), testosterone (male), prolactin, GH, and insulin-like growth factor 1. For this condition, thyroid replacement therapy is monitored by checking the levels of FT4 and FT3.[45]
Other causes include drug use (e.g., phenytoin, rifampin, carbamazepine, barbiturates) and assay error when interfering substances are present.[Figure caption and citation for the preceding image starts]: Differentiating causes of normal TSH and low free T4 (FT4) and/or free T3 (FT3)From the collection of Dr Sheikh-Ali [Citation ends].

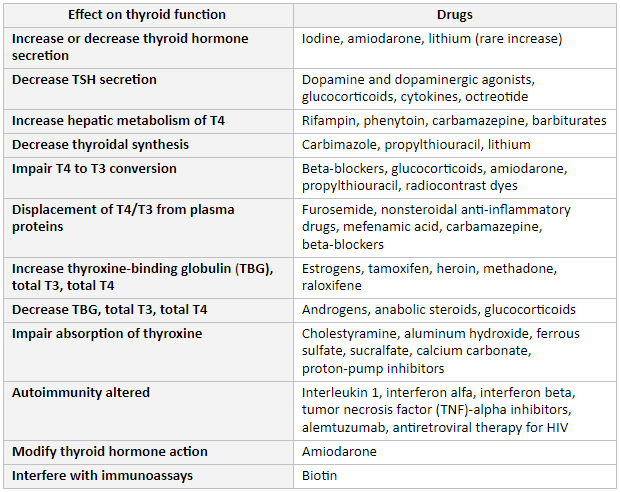
Many commonly used medications affect thyroid function.[26] Therefore, the possible effect of these drugs both on the results of TFTs and on the effectiveness of treatment should always be considered in decisions regarding patient care.
Iodine, amiodarone, or lithium increase or decrease thyroid hormone secretion.
Dopamine and its agonists, as well as glucocorticoids, cytokines, or octreotide, decrease TSH secretion.
Rifampin, phenytoin, carbamazepine, or barbiturates increase hepatic metabolism.
Carbimazole, propylthiouracil, or lithium decrease thyroid hormone synthesis.
Beta-blockers, glucocorticoids, amiodarone, propylthiouracil, or radiocontrast dyes impair T4 to T3 conversion.
Furosemide, nonsteroidal anti-inflammatory drugs (NSAIDs), mefenamic acid, carbamazepine, or beta-blockers displace T4/T3 from plasma proteins.
Estrogens, tamoxifen, heroin, methadone, or raloxifene increase thyroxine-binding globulin (TBG), total T4, and total T3 levels.
Androgens, anabolic steroids, or glucocorticoids decrease TBG, total T4, and total T3 levels.
Cholestyramine, aluminum hydroxide, ferrous sulfate, sucralfate, calcium carbonate, or proton-pump inhibitors impair absorption of thyroxine.
Interleukin-1, interferon alfa, interferon beta, tumor necrosis factor (TNF)-alpha inhibitors, alemtuzumab, and antiretroviral therapy for HIV are associated with risk of autoimmune thyroid dysfunction.[18]
Immune checkpoint inhibitors, including those that inhibit cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) receptor, can cause irreversible hypothyroidism. Hypophysitis leading to central hypothyroidism occurs more frequently in patients taking CTLA-4 inhibitors, whereas primary thyroid dysfunction is seen more often with PD-1 inhibitors.[19]
Amiodarone may also modify thyroid hormone action.
Biotin can interfere with immunoassays and cause falsely decreased TSH and increased free T4.[2] Patients should therefore be asked about their intake of biotin supplements.[34][Figure caption and citation for the preceding image starts]: Drug effects on the thyroidFrom the collection of Dr Sheikh-Ali [Citation ends].
Use of this content is subject to our disclaimer