Etiology
The Philadelphia chromosome is present in >95% of cases of chronic myeloid leukemia (CML) and is a hallmark of this disease.
Little is known about the underlying pathophysiologic changes that lead to the transformative mutation preceding the Philadelphia chromosome.
Patients with CML treated with older alkylating agents (primarily busulfan) may develop blast crisis quickly.[13]
Exposure to ionizing radiation is associated with increased risk for CML; risk appears to be greater with exposure to higher radiation doses.[14][15]
Pathophysiology
The Philadelphia chromosome arises from reciprocal translocation of chromosomes 9 and 22, juxtaposing the proto-oncogene c-abl from chromosome 9 to the breakpoint cluster region (BCR) in chromosome 22. The resulting BCR::ABL1 fusion gene transcribes a constitutively activated tyrosine kinase, which activates downstream cellular signal transduction pathways, leading to CML.
Blast crisis refers to the transformation of CML from the chronic or accelerated phase to the blast phase as a result of continued BCR::ABL activity.[Figure caption and citation for the preceding image starts]: BCR::ABL1 translocationFrom the collection of Dr Han Myint; used with permission [Citation ends].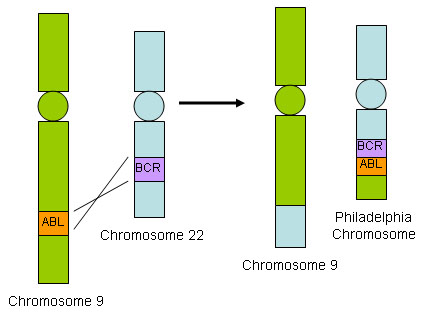
Transformation to accelerated or blast phase is characterized by the accumulation of molecular and chromosomal abnormalities, although the mechanisms involved are unclear. Increased BCR::ABL tyrosine kinase activity is thought to promote secondary molecular and cytogenetic changes leading to clonal evolution and cell differentiation arrest. The mechanisms involved are complex and may include oncogene activation, loss of tumor suppressor genes, blockade of cell differentiation, malfunction of apoptosis, telomere theory, and abnormal epigenetic modification.[16][17][18]
Advanced stage CML develops with increasing genomic instability, the accumulation of genetic abnormalities, and resistance to treatment. Blast phase occurs with cell differentiation arrest and enhanced self-renewal of leukemic progenitor cells, resulting in rapid proliferation of immature myeloid or lymphoid blast cells.[17] Among the clinical sequelae are fatigue, malaise, failure to thrive, bleeding, weight loss, night sweats, and bone pain. This contrasts with chronic and accelerated phases of CML, which are less symptomatic.
Additional chromosomal abnormalities (ACAs) and somatic mutations are acquired during the course of CML. High-risk ACAs (e.g., i(17)(q10), −7/del7q, and 3q26.2 rearrangements) and high-risk somatic mutations (e.g., ASXL1 and ABL1 kinase domain mutations) are associated with progression to blast phase and poor prognosis.[19][20] Some mutations and ACAs are common to both myeloid and lymphoid blast crisis (e.g., ABL1 kinase domain mutations, RUNX1); others are more commonly associated with myeloid phenotype (e.g., ASXL1 and TP53 mutations) or lymphoid phenotype (e.g., IKZF1 mutation and −7/del7q ACA).[17]
Classification
Subtypes of blast crisis
Myeloid predominant (approximately 70% to 80% of cases):[4][5]
Behaves like acute myeloid leukemia.
Lymphoid predominant (approximately 20% to 30% of cases):[4][5]
Behaves like acute lymphoblastic leukemia.
Additional cytogenetic abnormalities may be present.
Has the best prognosis among the different subtypes.
Mixed lineage (rare)
Biphenotypic.
Use of this content is subject to our disclaimer