Etiology
Pathophysiology
Overview of factors affecting neutrophil maturation, migration, and demargination.
The peripheral circulation of neutrophils, which is included in the total leukocyte count, consists mostly of mature neutrophils in transit from the bone marrow to peripheral tissues. This transit is short and depends on signaling through the chemokine receptor CXCR4 on the surface of neutrophils.[1] Only 5% of the total number of neutrophils is present in the circulation at any one time, as the neutrophil lifespan includes 9 days within the bone marrow, and 1 to 4 days in peripheral tissues, with only 3 to 6 hours spent in the circulation.
Bone marrow production of cells within the myeloid lineage is stimulated by interleukin 3 and granulocyte macrophage colony-stimulating factor (GM-CSF). Granulocyte colony-stimulating factor (G-CSF) is responsible for catalyzing differentiation of early myeloid cells into mature neutrophils.
Multiple signals induce neutrophil migration to the peripheral circulation from the marrow, including corticosteroids, endotoxins, complement-derived leukocyte-mobilizing factor, C5a chemoattractant, tumor necrosis factor-alpha, and androgens. When triggered, release of neutrophils from bone marrow storage triples circulating neutrophils within 4 hours.
In addition to neutrophils contained within the bone marrow, circulation, and peripheral tissues, a moderate population adheres to the vascular endothelium and enters the circulation on demargination. Release of catecholamines, including epinephrine, results in almost immediate demargination and subsequent doubling of the peripheral neutrophil count.[4]
Therefore, neutrophilia may be a result of:
Increased mature myeloid cell production
Increased demargination (process of neutrophils entering the peripheral circulation from areas of intravascular marginated polymorphonuclear cell pools), or
Decreased egress (outward migration) of neutrophils from the peripheral circulation into the tissues.
A spurious laboratory value should be ruled out before further investigation.
Hematopoiesis within the bone marrow, as well as in extramedullary sites (liver, thymus, and spleen), is influenced by systemic conditions that may inhibit (e.g., chemotherapy) or stimulate cell production (e.g., infection). An increased neutrophil count due to increased production may be classified as primary or secondary neutrophilia. Primary neutrophilia is the result of a pathologic process affecting the bone marrow; secondary neutrophilia occurs when the bone marrow responds appropriately to a systemic stimulus for increased neutrophil production.
Spurious neutrophilia
Before initiating an intensive investigation into the cause of a reported neutrophilia, it is first important to confirm the validity of the laboratory value. Neutrophilia may be a laboratory aberrancy secondary to platelet clumping or cryoglobulinemia. Evaluation of the peripheral blood smear allows either diagnosis to be rapidly excluded.
Platelet clumping
If the peripheral blood sample contains platelet clumps, automated cell counters may mistake platelet clumps for white blood cells, most often lymphocytes. The overall effect is a modest increase in WBC count, typically reported with an associated thrombocytopenia.
In the setting of adequate sample anticoagulation, spurious neutrophilia and thrombocytopenia may occur if a patient has ethylenediaminetetraacetic acid (EDTA)-dependent agglutinins. This phenomenon, also known as pseudoleukocytosis, occurs in 0.1% of patients and requires reassessment of the platelet and total leukocyte count in a non-EDTA blood collection tube.
Cryoglobulinemia
If the peripheral blood sample is cooled below room temperature and contains cold-insoluble plasma proteins, a temperature-dependent increase in the WBC count and platelet count will be observed at ≤30°C. Precipitated cryoglobulin particles will return to solution if the sample is warmed to body temperature. Mean cell hemoglobin may also be elevated, which corrects with warming.
Increased production: primary neutrophilia
In primary neutrophilia, production is upregulated as a direct result of a primary neoplastic process within the bone marrow or an inherited condition.
Factors supporting a neoplastic etiology for neutrophilia include sustained WBC count >50,000/microliter, left shift of the neutrophil lineage, basophilia or eosinophilia, dysplasia, and abnormal red blood cells such as dacrocytes (teardrop-shaped red blood cells).[5]
Myeloproliferative neoplasm (MPN)
This is a spectrum of hematologic diseases, including chronic myeloid leukemia (CML), chronic neutrophilic leukemia (CNL), primary myelofibrosis (proliferative phase), essential thrombocythemia, polycythemia vera, and unclassifiable MPN, all of which can progress to acute leukemia. Among a subset of patients, including those with essential thrombocythemia, the degree of elevation in the total leukocyte count may predict disease risk. Evidence of immature cells in the peripheral circulation may support the diagnosis of MPN.
CNL is associated with a persistent mature neutrophilia with only rare neutrophil precursors in the peripheral blood. CNL is associated with activating mutations of the CSF3R gene, most commonly T618I.[6] If considering the diagnosis of CNL, plasma cell myeloma (multiple myeloma) should be excluded.
CML is supported diagnostically by evidence of the Philadelphia chromosome, translocation t(9;22)(q34.1;q11.2), resulting in the BCR-ABL1 fusion gene. Patients exhibit a leukocytosis of 12,000 to 1,000,000/microliter due to a neutrophilia with left-shifted granulocytes (an increase in immature granulocytes in the peripheral circulation), typically with elevated percentages of segmented neutrophils and myelocytes (an immature myeloid cell derived from promyelocytes and giving rise to metamyelocytes not seen normally in the circulation), basophilia, eosinophilia, and often thrombocythemia. Diagnosis is also supported by a low leukocyte alkaline phosphatase score. [Figure caption and citation for the preceding image starts]: BCR-ABL1 translocationFrom the collection of Dr Han Myint and Dr Robert Chen; used with permission [Citation ends].

Myelodysplastic/myeloproliferative neoplasm
This is a spectrum of overlapping syndromes of dyspoiesis and increased cell proliferation, including chronic myelomonocytic leukemia, atypical CML, and unclassifiable myelodysplastic/myeloproliferative neoplasm. Evidence of immature cells in the peripheral circulation along with dysplasia may support the diagnosis of myelodysplastic/myeloproliferative neoplasm.
Acute myeloid leukemia (AML)
Specific subtypes of AML can present with neutrophilia. The presence of blasts in the peripheral blood should prompt a workup to exclude acute leukemia. Over 20% blasts in either the blood or bone marrow is diagnostic of AML. AML is suggested by an elevated total leukocyte count with evidence of immature myeloid cells in the circulation, and associated anemia and thrombocytopenia.
Hereditary neutrophilia[7]
This is a rare autosomal dominant syndrome with a phenotype that does not lead to increased infections or a leukemic predisposition. It is associated with platelet dysfunction leading to a bleeding diathesis.
Diagnostic findings include total leukocyte count >20,000/microliter, elevated leukocyte alkaline phosphatase, normal neutrophil function, and positive CD18/CD11b expression on the neutrophil surface.
Pelger-Huet abnormality
This is a dominantly inherited disorder of neutrophil differentiation due to lamin B-receptor gene mutations. Although neutrophil nuclei appear to have 2 lobes with a single connecting strand, no functional deficit occurs secondary to the morphologic abnormality.
Pelgeroid (pseudo-Pelger-Huet) cells outside of the inherited disorder are also seen in myelodysplastic syndrome, and less commonly, in active infection, acute and chronic leukemia, or with medications such as mycophenolate mofetil, colchicines, and sulfonamides. These drugs cause pelgeroid features in the neutrophil nuclei, and neutrophils retain their cytoplasmic granulation. In contrast, in myelodysplastic syndrome, hypolobation of neutrophils with pelgeroid features is typically accompanied by hypogranulation of the cytoplasm.
Amegakaryocytic thrombocytopenia
This condition presents during early childhood and is associated with an inherited low platelet count. The disease course is heterogeneous, and a subset may progress to AML.
Trisomy 21 (Down syndrome)
Infants and children with Down syndrome may have temporary leukemoid reactions of unclear significance in the setting of stress or inflammation. Transient abnormal myelopoiesis at birth may resemble AML.
Leukocyte adhesion deficiency
In addition to persistent leukocytosis and neutrophilia, recurrent infections are seen when neutrophil function is impaired. As neutrophils in this condition lack CD18, no leukocyte and beta-2 integrins remain. In patients with leukocyte adhesion deficiency type I, neutrophils are defective in chemotaxis, adherence, and phagocytosis, and there is a stimulus-dependent defect in neutrophil activation.
Familial cold autoinflammatory syndrome
This condition presents with a leukocytosis >25,000/microliter within 12 hours of exposure to the cold.
Chronic idiopathic neutrophilia
This condition is diagnosed if no evidence of pathology is revealed with a complete diagnostic evaluation, including bone marrow aspiration and biopsy, for neutrophilia.
Abdominal imaging should be considered, as congenital asplenia has been identified in a subset of patients with chronic idiopathic neutrophilia.
Increased production: secondary neutrophilia
In secondary neutrophilia, the elevated neutrophil count is due to increased bone marrow production as a response to a systemic stimulus, such as infection, inflammation, or a drug reaction. Some systemic conditions lead to increased hematopoiesis as an endogenous response.
Infection
Active infections, not limited to those of bacterial origin, often result in an elevated total leukocyte count with a left shift (an increase in immature granulocytes in the peripheral circulation) among patients of all ages, including preterm infants.[8] In the setting of severe sepsis, most commonly in older people and neonates, the total leukocyte count and neutrophil count may be decreased rather than increased.
Particular infectious diseases are associated with more elevated total leukocyte counts, such as those caused by Clostridiumspecies. A leukemoid reaction is a leukocytosis of >50,000/microliter, characterized by a neutrophilia with increased myeloid precursors secondary to a nonmalignant condition (e.g., secondary neutrophilia).
In response to infectious processes, epinephrine is released and induces demargination (process of neutrophils entering the peripheral circulation from areas of intravascular marginated polymorphonuclear cell pools) of neutrophils, which further augments the elevation in neutrophils.
Inflammation
Chronic and severe acute inflammatory conditions resulting in neutrophilia include Kawasaki disease, juvenile- and adult-onset rheumatoid arthritis, Crohn disease, ulcerative colitis, chronic granulomatous infections, and bronchiectasis. The presence of a neutrophilia typically predicts poor prognosis, and among patients with Crohn disease, risk of recurrence postsurgery is predicted by preoperative neutrophilia.[9]
Immunization may also lead to a secondary neutrophilia.
Anemia
Any process resulting in anemia, including hemolysis and blood loss, can cause increased hematopoiesis to compensate for a low hemoglobin, leading to secondary elevation of the total leukocyte, neutrophil, and platelet counts.
Microangiopathic hemolytic anemias include disseminated intravascular coagulation, thrombotic thrombocytopenic purpura (TTP), and hemolytic uremic syndrome (HUS). Diagnosis is suggested by microangiopathic changes to the red blood cells (fragmented red blood cells, reticulocytosis) and enlarged platelets. Half of patients with TTP or HUS have evidence of a neutrophilia or leukocytosis with a total leukocyte count >20,000/microliter. Some reports suggest neutrophilia is associated with a poor outcome in TTP and HUS.[10]
Solid tumor malignancy
Tumor-induced leukocytosis is a common phenomenon of unclear etiology. Neutrophilia may be present with or without solid tumor infiltration within the bone marrow. A subset of solid tumors, particularly colonic adenocarcinoma, has been shown to release growth factors that induce the secretion of, or mimic, granulocyte (G-CSF) and granulocyte macrophage colony-stimulating factors (GM-CSF).[11]
A neutrophil-to-lymphocyte ratio ≥4 is an adverse prognostic factor in patients with mesothelioma, pancreatic cancer, renal cell carcinoma, and colorectal cancer.[12]
Bone marrow infiltration
Infiltrative processes within the bone marrow such as tumor, fibrosis, or granulomatous disease may increase the neutrophil count, often with concurrent morphologic changes in circulating blood cells, including a leukoerythroblastic blood smear (left-shifted granulocytes and nucleated red blood cells) with teardrop-shaped red blood cells (dacrocytes).
To determine the etiology, a bone marrow biopsy is indicated.[Figure caption and citation for the preceding image starts]: Toxic granules in a patient receiving granulocyte colony-stimulating factor treatmentFrom the collection of T. George, MD [Citation ends].
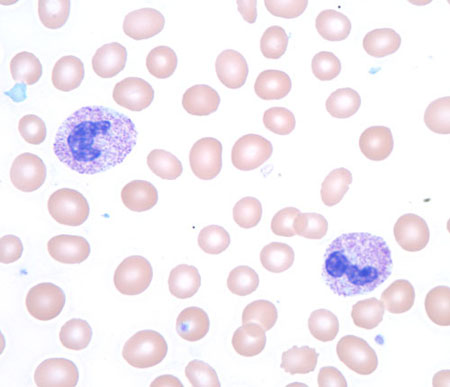 [Figure caption and citation for the preceding image starts]: Leukoerythroblastic peripheral blood smear with dacrocytesFrom the collection of T. George, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Leukoerythroblastic peripheral blood smear with dacrocytesFrom the collection of T. George, MD [Citation ends].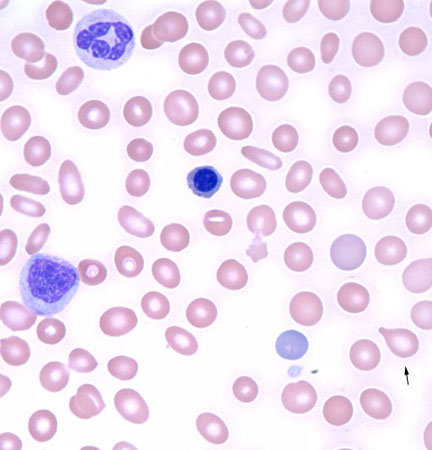
Chronic bone marrow stimulation
Chronic medical conditions inducing stimulation of the bone marrow as compensation for either anemia or thrombocytopenia result in secondary leukocytosis. These conditions include hemolytic anemias, such as sickle cell anemia, in which leukocytosis is commonly found in presentations with vaso-occlusive crises or infection.
Recovering bone marrow function may induce a transient leukocytosis with leukoerythroblastic changes following cytotoxic therapy such as chemotherapy. After completion of chemotherapy, a neutrophilia may be present until granulopoiesis adjusts to the withdrawal of cytotoxic treatment.
Heat stroke
Morphologic changes in circulating neutrophils and elevation of the total neutrophil count occur acutely with heat stroke.
Neutrophil segments appear smaller than normal with a botryoid appearance (having numerous rounded protuberances resembling a bunch of grapes).
Asplenia
Neutrophilia after resection of the spleen is common. Although a moderate degree of neutrophilia is transient, most patients will continue to have a chronically mild neutrophilia.
Conditions that result in functional asplenia, including sickle cell disease, congenital asplenia, veno-occlusive disease, and autoinfarction, also induce a chronic mild neutrophilia. Evidence of leukocytosis in sickle cell disease at a young age, outside the setting of infection, is prognostic of increased disease severity.
Sweet syndrome
This condition is characterized by an acute febrile neutrophilic dermatosis.[13]
The normal rate of hematopoiesis may be altered as a desired or adverse effect of some drugs, including:
Tobacco: the most common cause of mild neutrophilia. The absolute neutrophil count doubles among smokers of at least 2 packs per day, and a 25% rise in the total leukocyte count is seen in all smokers. The phenomenon occurs due to smoking-induced inflammatory changes, which require up to 5 years from cessation of smoking to normalize and for the total leukocyte and neutrophil counts to decline.[14]
GM-CSF: at a dose of 250 micrograms/m², neutrophil production within the bone marrow is not affected, but neutrophil migration from the peripheral blood to specific tissue sites is reduced while release of neutrophils from within the bone marrow is accelerated.[15] The duration of neutrophilia among healthy volunteers is 14 days.
G-CSF: daily dosing of G-CSF can result in a 12-fold elevation of the total leukocyte count. G-CSF therapy seems to improve neutrophil function, with increased integrin expression, neutrophil cellular elastase activity, and plasma elastase antigen levels.
Lithium: enhances granulopoiesis by increasing production of GM-CSF and G-CSF. Before the development of recombinant stimulating factors, lithium was used for this purpose, although the response was variable.[16]
All-trans retinoic acid (ATRA) or arsenic trioxide: 50% of patients with acute promyelocytic leukemia (AML-M3 by French-American-British classification) develop a transient leukocytosis due to therapy with ATRA or arsenic trioxide. Both therapies may result in differentiation syndrome, which occurs in 25% of patients. Differentiation syndrome manifests with fever, weight gain, peripheral and pulmonary edema, pleural and pericardial effusions, hypotension, and renal dysfunction.
Obesity
A subgroup of obese patients has a persistent leukocytosis. This is likely due to the production of inflammatory cytokines including interleukin-6, tumor necrosis factor-alpha, interleukin-1, and interleukin-8 by adipose tissue.[17]
In addition, adipocytes produce leptin, which can act as a granulocyte colony-stimulating factor.[18]
Increased demargination
Demargination is the process of neutrophils entering the peripheral circulation from areas of intravascular marginated polymorphonuclear cell pools. Similar to the conditions underlying increased bone marrow production of neutrophils, demargination may occur in response to stress and certain drugs.
Stress-induced demargination
Physiologic and psychological stress can lead to a mild neutrophilia. Conditions associated with anxiety and stress (anxiety disorder, post-traumatic stress disorder, panic disorder, and depression) have been shown to result in neutrophil demargination.[19]
Among patients presenting with suspected myocardial infarction, those with a total leukocyte count >9000/microliter were 4.5 times more likely to have confirmed myocardial infarction than patients with a total leukocyte count <6000/microliter.[20] Leukocytosis is an independent predictor of mortality among patients with acute myocardial infarction.[21][22]
Increased cardiac output in exercise leads to significant mechanical and flow-related forces that dislodge neutrophils sequestered in the pulmonary vasculature, causing demargination. Thus, a delayed neutrophilia often occurs due to increased release of neutrophils from within the bone marrow.
Other causes of stress-induced transient neutrophilia are muscular convulsions (e.g., tonic-clonic seizure), emesis, ovulation, obstetric conditions (preeclampsia, and spontaneous or cesarean delivery), carbon monoxide poisoning, a thyroid storm, and trauma (thermal burn, electric shock, surgery, and snake bites).
Drug-induced demargination
Epinephrine: beta-agonists induce acute elevations in the neutrophil count as a result of neutrophil release from marginated stores.[4]
Corticosteroids: total leukocyte and neutrophil counts increase moderately after corticosteroid use at or above the equivalent dose of prednisone 40 mg daily.[23] Demargination due to corticosteroids results from the decreased adhesive capacity of neutrophils and an increased release of neutrophils from within the bone marrow.
Antidepressants: the mechanism is unclear.
Decreased egress
Normal trafficking of mature myeloid cells depends on natural egress (outward migration) of neutrophils from the circulating intravascular space into the peripheral tissues. This process may be inhibited by medications such as corticosteroids.
Use of this content is subject to our disclaimer