Aetiology
Your Organisational Guidance
ebpracticenet urges you to prioritise the following organisational guidance:
Chronisch nierlijden (multidisciplinaire aanpak)Published by: WORELLast published: 2017GPC pluridisciplinaire sur la néphropathie chronique (IRC)Published by: Groupe de travail Développement de recommandations de première ligneLast published: 2017Creatinine is an amino acid breakdown product of creatine and phosphocreatine. It is generated almost exclusively in skeletal muscle (90%) at a fairly constant rate, and is freely filtered through the glomerulus. In addition, 5% to 10% of creatinine is secreted by the proximal tubules.
Laboratory measurement of creatinine
Serum creatinine is commonly measured by alkaline picrate (Jaffe reaction), enzymatic, and high-performance liquid chromatography (HPLC) methods. Subsequent to recommendations for improving creatinine measurement, these methods are standardised to the isotope dilution mass spectrometry (IDMS) method.[1]
Point-of-care testing (POCT) is now commonly available in healthcare settings.
Isotope dilution mass spectrometry (IDMS)
IDMS is the diagnostic standard.
It is highly specific and offers the most accurate results for serum creatinine, but is available only in selected laboratories.
The Jaffe method
Commonly used but is subject to interference by a range of substances. Glucose and ketones can interfere with the assay, leading to an overestimation of serum creatinine in patients with diabetic ketoacidosis.[2]
Delayed sample receipt and centrifugation can lead to significant increases in measured creatinine using the Jaffe method.[3]
Guidelines recommend moving towards enzymatic methodology due to the poor specificity of Jaffe creatinine methods. Differences between Jaffe and enzymatic serum creatinine results can exceed the recommended 5% target, especially at concentrations 100 micromol/L (<1.13 mg/dL).[4]
HPLC methods
Have better specificity than the Jaffe and enzymatic methods, and are less prone to interference. However, measurement errors can occur due to variability in calibration, and to random measurement errors.[1]
Combining HPLC with IDMS provides highly accurate results, but is not available in most centres.
POCT enzyme-based serum creatinine measurement
Appears to be sufficiently accurate for clinical use in critically ill patients.[5]
Creatinine POCT can be used for screening patients at risk for contrast-induced acute kidney injury (AKI) prior to contrast-enhanced diagnostic imaging.[6][7]
Dry blood spot sample
An innovative method of creatinine measurement, especially for screening chronic kidney disease (CKD).
This method has a sensitivity of 96% and specificity of 55%.[8]
Estimated GFR (eGFR) and creatinine clearance using serum creatinine
The most accurate method for calculating GFR is by measuring the clearance of exogenous filtration markers, such as iothalamate, iohexol, or inulin. However, this is expensive and requires exposure to radiation and compliance with strict regulatory guidelines. In practice, therefore, creatinine clearance is used to estimate GFR. Creatinine is freely filtered, has minimal tubular secretion and absorption, is simple and inexpensive to measure from random blood samples, and has relatively good accuracy. A rise in serum creatinine is used as a marker of reduced GFR. It varies inversely with GFR, but the relationship is not linear.
The use of serum creatinine as an indirect filtration marker is limited by the following factors:
Biological variability
Bias and non-specificity affecting creatinine measurement
Medication effects
Nutrition
Alterations in circulating serum creatinine produced by non-renal disease states
Differences in GFR range and creatinine production in healthy people compared with people with CKD.
As a result of these confounding factors, there is a risk of overestimating the GFR, and the magnitude of the overestimation is not predictable.[9]
Equations for estimating GFR
Equations for estimating GFR from serum creatinine levels are mainly used for staging CKD and should not be used to interpret acute increases in serum creatinine. Correction factors for black people have generally been derived from studies in African-American people. UK guidelines do not recommend using correction factors for ethnicity.[10]
Available equations include:
Cockcroft-Gault formula[11] [ Creatinine Clearance Estimate by Cockcroft-Gault Equation Opens in new window ]
Four-variable Modification of Diet in Renal Disease (MDRD) formula[2] [ Glomerular Filtration Rate Estimate by the Abbreviated MDRD Study Equation (SI units) Opens in new window ]
Isotope dilution mass spectrometry (IDMS)-traceable 4-variable MDRD equation (MDRD-IDMS)[12] [ Glomerular Filtration Rate Estimate by the IDMS-Traceable MDRD Study Equation Opens in new window ]
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation[13] [ Glomerular Filtration Rate Estimate by CKD-EPI Equation Opens in new window ]
Mayo Clinic equation[14]
Lund-Malmo revised (LMR)[15]
Full age spectrum (FAS) (can be used in children and adults of all ages)[16]
Berlin initiative study 1 and 2 (BIS1, BIS2)[17]
European Kidney Function Consortium equations[18]
[Figure caption and citation for the preceding image starts]: Mayo Clinic equationFrom: Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004 Dec 21;141(12):929-37. Bjork J, Back SE, Sterner G, et al. Prediction of relative glomerular filtration rate in adults: new improved equations based on Swedish caucasians and standardized plasma-creatinine assays. Scand J Clin Lab Invest. 2007;67(7):678-95. Used with permission. [Citation ends].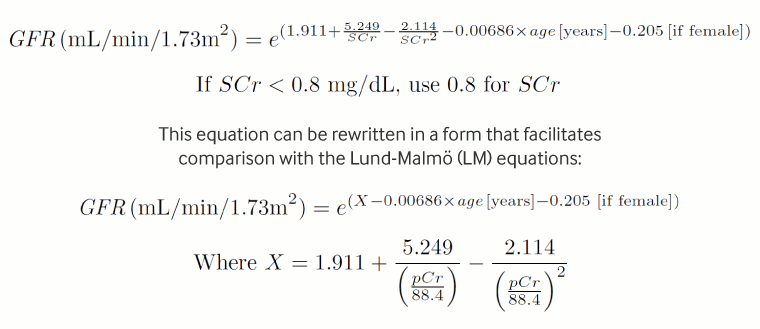 [Figure caption and citation for the preceding image starts]: Lund-1 equation without body weight measureFrom: Bjork J, Back SE, Sterner G, et al. Prediction of relative glomerular filtration rate in adults: new improved equations based on Swedish caucasians and standardized plasma-creatinine assays. Scand J Clin Lab Invest. 2007;67(7):678-95. Used with permission. [Citation ends].
[Figure caption and citation for the preceding image starts]: Lund-1 equation without body weight measureFrom: Bjork J, Back SE, Sterner G, et al. Prediction of relative glomerular filtration rate in adults: new improved equations based on Swedish caucasians and standardized plasma-creatinine assays. Scand J Clin Lab Invest. 2007;67(7):678-95. Used with permission. [Citation ends].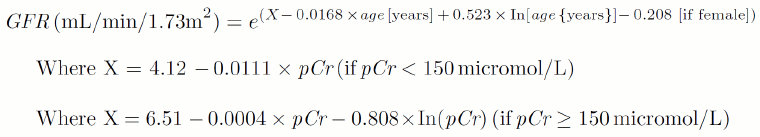
A comparison of GFR estimation equations reveals that the MDRD formula performs well in populations with a low range of GFR, and often outperforms the Cockcroft-Gault equation. Both equations have lower precision in high-GFR populations, and the MDRD equation underestimated the GFR in some studies. The Cockcroft-Gault, simplified MDRD, and CKD-EPI equations perform poorly in critically ill patients, and are not recommended in the intensive care unit setting.[19][20]
An evaluation of four different eGFR equations (CKD-EPI, LMR, FAS, BIS1) found that none was diagnostically superior in adults over 65 years of age with varying degrees of kidney impairment.[21]
Pitfalls in the use of serum creatinine and eGFR values
The rate of rise of serum creatinine (creatinine kinetics) is dependent on baseline GFR. In computer simulation models of creatinine kinetics, time to reach a 50% increase in serum creatinine was 10 hours when baseline GFR was normal versus >60 hours when GFR was <30 mL/min (CKD stage 4), contributing to a delay in the recognition of acute kidney injury by serum creatinine criteria.[22]
Creatinine kinetics is influenced by volume kinetics. Serum creatinine decreases by about 20% following crystalloid infusion and 40% following colloid infusion, and may take 4-12 hours to return to baseline. Uncorrected, this can lead to delay in the diagnosis of AKI.[23]
Acute changes in serum creatinine should be considered in the setting in which they take place. In patients with heart failure, various patterns of serum creatinine changes have been observed (bump, sustained increase, dip, sustained decrease, dip followed by bump, bump followed by dip, or no change). However, in one observational analysis, none of the patterns predicted diuretic responsiveness, death, or cardiovascular or renal rehospitalisation.[24]
In certain conditions, elevated serum creatinine is not the primary concern. In heart failure (cardiorenal syndromes), patients with sustained decongestion and worsening renal function had better outcomes than those with congestion and worsening renal function.[25]
eGFR overestimates GFR in low GFR states (due to increased tubular secretion of creatinine).
eGFR is inaccurate in high GFR states (due to the lack of eGFR values above 60 mL/minute/1.73 m²).
Studies suggest that the Cockroft-Gault and MDRD formulas correctly assigned only 64% and 62% of patients, respectively, to their actual CKD classification GFR group. Based on US National Health and Nutrition Examination Surveys (NHANES 1988-1994; 1999-2004) and US population census data (2000), this suggests that around 10 million people (38%) may have been misclassified in the US.[26][27][28]
Serum creatinine and eGFR may not be equivalent in all clinical situations. In contrast media-induced acute kidney injury, an increase in serum creatinine, but not eGFR, was predictive for long-term mortality, with a threshold of 44.2 micromol/L (0.5 mg/dL) or more indicating worse prognosis.[29]
Prediction of renal function is often inaccurate in individuals with class 2/3 obesity (BMI ≥35 kg/m²). Body cell mass GFR (BCM GFR) predicts creatinine clearance more accurately than traditional formulas in this patient population.[30]
The inclusion of race in eGFR algorithms results in higher reported eGFR in black patients. The MDRD equation and the CKD-EPI equation (that included a larger number of black patients in the study population) report higher eGFR (by a factor of 1.210 and 1.159, respectively) if the patient is identified as black.[31] There are concerns that the higher eGFR reported using algorithms that include race risks worsening health inequities in affected populations by delaying specialist referral or assessment for kidney transplant.[31]
In contrast to North American guidelines, UK guidelines recommend using the CKD-EPI equation to estimate GFR from serum creatinine in adults; and since 2021 they no longer recommend using correction factors for adults of African-Caribbean or African family origin.[10]
The European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) recommends either the Lund-Malmo or the European Kidney Function Consortium (EKFC) equations, as they were developed and validated in European cohorts.[32] The EKFC eGFR equation depends on adjustments to an individual's serum creatinine value that derives from median values in healthy populations across the age spectrum (from infants to older adults), sex, and race.[33][34]
The controversy regarding the optimal method to estimate GFR for disease detection and monitoring is unresolved. Comparisons of GFR estimation, using the CKD-EPI equation and other creatinine-based equations in different populations found that these equations are not applicable to all populations and need to be individually validated prior to their routine use.[35][36][37][38]
The UK Medicines and Healthcare products Regulatory Agency advises that eGFR is an appropriate measure of renal function for most patients but recommends using the Cockroft-Gault formula to estimate creatinine clearance when determining dose adjustments for:[39]
direct-acting oral anticoagulants
nephrotoxic drugs (e.g. vancomycin, amphotericin B)
drugs that are largely renally excreted and have a narrow therapeutic index (e.g. sotalol, digoxin)
patients aged ≥75 years; eGFR considerably overestimates kidney function for prescribing in patients >65 years[40]
patients with body mass index ≤18 kg/m² or ≥40 kg/m²
Renal function and dose adjustments should be reviewed frequently in situations where eGFR or creatinine clearance change rapidly, for example in patients with AKI.
Cystatin C and serum creatinine
Cystatin C is an alternative marker of kidney function and is used as a confirmatory test for eGFR when more precise estimates for clinical decision making are required. Creatinine is generated mostly in skeletal muscles whereas cystatin C is produced by all nucleated cells. Cystatin C is fully catabolised in the proximal renal tubule following glomerular filtration but is not returned to blood. Unlike serum creatinine, serum cystatin C is less affected by gender, age, race, protein intake, and muscle mass but is influenced by smoking, inflammation, adiposity, thyroid diseases, malignancy, and glucocorticoids.
When serum creatinine- and cystatin C-based eGFRs are similar, either biomarker provides an accurate eGFR. However, where there are large differences, eGFR based on both serum creatinine and cystatin C is more accurate than either biomarker alone.[41]
Although data are limited, one retrospective study reports that eGFR based on cystatin C can have a substantial effect on CKD staging. Compared with creatinine eGFR, cystatin C eGFR led to reclassification to a more advanced CKD stage in 27% of patients, a less advanced stage in 7%, and no change in 66% of patients.[42]
The EKFC cystatin C-based eGFR equation has the same mathematical form as the EKFC creatinine-based eGFR equation, but has a scaling factor for cystatin C that does not need to be adjusted for race or sex. In one cross-sectional analysis of patient data from multiple centres in Europe, US, and Africa, it was found to be more accurate in assessing GFR than any of the three CKD-EPI equations or the revised Lund-Malmo equation.[18]
Kinetic estimated GFR
eGFR is based on the assumption of a steady-state creatinine concentration. However, AKI is a non-steady state and eGFR in this situation is unreliable.
Kinetic eGFR (KeGFR) is an estimate of immediate biomarker clearance based on two serum creatinine measurements at different timepoints. It reflects dynamic changes in renal function and can be determined from routine tests carried out in acutely ill patients.[43] KeGFR calculator Opens in new window KeGFR may be an important predictor of mortality. However, in one retrospective analysis, there was not a good correlation between AKI severity and the worst achieved KeGFR (<30 mL/min).[44]
KeGFR has also been shown to improve prediction of dialysis and recovery after renal transplant.[45]
Acute kidney injury
There is considerable debate regarding the magnitude of serum creatinine increase that constitutes acute kidney injury (AKI).
Consensus defines AKI as any of the following:[46][47][48]
Abrupt reduction in kidney function, defined as an absolute increase in serum creatinine of ≥26.5 micromol/L (≥0.3 mg/dL) within 48 hours
An increase in serum creatinine to 1.5-fold from baseline within the last 7 days
A reduction in urine output (documented oliguria of <0.5 mL/kg/hour for >6 hours).
AKI is staged as follows:
Stage 1: increase in serum creatinine >26.5 micromol/L (>0.3 mg/dL) or 150% to 200% of baseline values.
Stage 2: increase of 200% to 300% (2- to 3-fold) of baseline values.
Stage 3: >300% (3-fold) increase in serum creatinine from baseline.
Use of serum creatinine to detect and assess the severity of AKI is limited. Serum levels are influenced by many factors, so the absolute level does not reflect the severity of the underlying kidney damage. eGFR is based on the assumption of a steady-state creatinine concentration, but AKI is a non-steady state.
Rises in serum creatinine after marked injury take 12 to 24 hours to occur and do not detect early-stage damage. In addition, creatinine kinetic studies have shown that the time to reach a 50% increase in serum creatinine is directly related to baseline kidney function and ranges from 4 hours (normal kidney function) to 27 hours (in stage 4 chronic renal failure).[22]
An alternative definition of AKI that incorporates absolute changes in serum creatinine over a 24- to 48-hour period has been proposed.[22]
KeGFR is an estimation of creatinine clearance that is calculated from two serum creatinine concentration measurements taken at different timepoints. It has an emerging role in risk-stratifying patients with rapidly changing renal function. Changes in KeGFR predict AKI and the need for renal replacement therapy more accurately than the MDRD equation.[49] KeGFR calculator Opens in new window
Chronic kidney disease
Both international and UK guidelines define CKD as structural or functional abnormalities of the kidney for >3 months with implications for health indicated by at least one of the following:[10][50]
GFR <60 mL/minute/1.73 m² for ≥3 months, with or without kidney damage.
Clinically important albuminuria (AER ≥ 30 mg/24 hours; ACR ≥30 mg/g [≥3 mg/mmol])
Urine sediment abnormalities
Electrolyte and other abnormalities due to tubular disorders
Abnormalities detected by histology
Structural abnormalities detected by imaging
History of kidney transplantation.
KDIGO (Kidney Disease Improving Global Outcomes) and NICE (UK National Institute for Health and Care Excellence) guidelines classify CKD stages according to cause, GFR, and albuminuria (CGA).[10][50]
eGFR categories (where G denotes the GFR category) are:
G1: kidney damage with normal or increased GFR, ≥90 mL/minute/1.73m²
G2: kidney damage with mild decrease in GFR, 60 to 89 mL/minute/1.73m²
G3a: kidney damage with moderate decrease in GFR, 45 to 59 mL/minute/1.73m²
G3b: kidney damage with moderate decrease in GFR, 30 to 44 mL/minute/1.73m²
G4: kidney damage with severe decrease in GFR, 15 to 29 mL/minute/1.73m²
G5: kidney failure (end-stage kidney disease), with GFR <15 mL/minute/1.73m².
Albuminuria categories (where A denotes the ACR category) are:
A1 (normal to mildly increased): albumin excretion rate (AER) <30 mg/24 hours, albumin-to-creatinine ratio (ACR) <3 mg/mmol (<30 mg/g)
A2 (moderately increased): AER 30-300 mg/24 hours, ACR 3-30 mg/mmol (30-300 mg/g)
A3 (severely increased): AER >300 mg/24 hours, ACR >30 mg/mmol (>300 mg/g).
For example, a person with an eGFR of 55 mL/minute/1.73 m² and an ACR of 40 mg/mmol has CKD G3aA3.
The diagnosis should be made following two measurements of eGFR at least 90 days apart.[10] Using a single screening test overestimated the prevalence of CKD by 25% in one primary care cohort study of people aged over 60 years.[51]
It is unclear whether estimation of GFR adds useful information to the serum creatinine measurement when determining CKD severity, or for guiding treatment. An increasing portion of serum creatinine is excreted by tubular secretion rather than glomerular filtration in advanced CKD, contributing to gross overestimation of GFR. Extra-renal secretion of serum creatinine is also increased, so the uptake of creatine generated by bacterial breakdown of creatinine in the gut, normally a negligible source of creatine, becomes significant.
Prognostic value of elevated creatinine
Mild increases in in-hospital serum creatinine have been associated with short-term mortality, progression to CKD, and accelerated progression to end-stage renal disease. Mild serum creatinine increases present a higher long-term mortality risk, especially in those with partial renal recovery.[52][53][54]
While findings from observational studies suggest that minimal and/or transient elevations in serum creatinine predict poor prognosis, one meta-analysis of placebo-controlled trials found no appreciable effect on CKD, or mortality, months after mild to moderate (often temporary) elevations in serum creatinine.[55]
Patients with chronically elevated serum creatinine (i.e., impaired baseline renal function) have a higher risk for AKI during hospital stays and are more often dialysis-dependent at hospital discharge than those without.[52][53][54][56][57][58][59] Chronically elevated serum creatinine has been linked to progression of CKD, increased mortality, and post-operative complications following cardiac surgery. Elevated serum creatinine after endovascular aneurysm repair has been reported to be a significant and strong predictor of post-operative mortality and complications.[60]
Factors influencing the generation of creatinine
Elevated serum creatinine is commonly associated with reduced GFR, but levels can also be elevated due to several other factors.
Muscle mass
The amount of creatinine the body produces each day depends on the person's muscle mass: a young, muscular man produces more creatinine than a petite, older woman. Failure to consider variations in creatinine production due to differences in muscle mass between individuals may lead to misinterpretation of serum creatinine levels. For example, a serum creatinine value in the reference range in a young, healthy person reflects a different GFR value than an identical serum creatinine value in an older person.
Low serum creatinine in certain muscle-wasting conditions, malnutrition, and amputation does not exclude an underlying renal dysfunction.
In obesity, excess mass is fat and does not contribute to increased creatinine generation.
Because muscle mass normally changes very little, creatinine is usually produced at about the same rate every day in each person.
Age and sex
Females and older people usually have less muscle mass and, consequently, lower production of creatinine, and lower serum creatinine than males and younger people. These age and sex differences are taken into account by most equations that estimate GFR.
The likelihood of CKD in older people and in women, and the risk of adverse outcomes, is inflated by equations that use patient demographics to estimate creatinine generation. In one study, the use of equations that used age to estimate creatinine generation were associated with a higher likelihood of CKD in older patients, and equations that used sex were associated with a higher likelihood of CKD in women.[61]
It is important to note that elevation of serum creatinine is not a normal feature of ageing, and is a pathological finding that needs further attention.
Race
The effect of race on serum creatinine levels is not clear. Even though serum creatinine concentrations are significantly higher in black than in non-black patients, these differences are not readily explained by differences in nutritional status or body composition.[62]
Nutrition
The creatine content of raw meat is 4 to 5 g/kg. Cooking meat converts creatine to creatinine, which is readily absorbed from the gastrointestinal tract into the circulation. The transient increase in serum creatinine can be the source of up to 30% of excreted creatinine.[63]
A vegetarian diet is associated with decreased generation of creatinine.
Creatine is often taken as a supplement to boost muscle mass and increase athletic performance. Typically, 20 to 30 g/day of creatine is given for 5 to 7 days in the loading phase, followed by 2 to 5 g/day in the maintenance phase.
Creatine supplementation increases phosphocreatine levels in the muscles (up to 20%), but only minimally affects serum creatinine concentrations and renal function in young healthy adults. Prolonged intake of creatine supplementation of >10 g/day may increase serum creatinine concentrations, but is unlikely to affect estimates of creatinine clearance.[64][65] Upon discontinuation of supplemental creatine, muscle creatine concentrations and urinary creatinine excretions return to baseline values in 3 to 4 weeks.
Intra- and inter-patient variability
Plasma creatinine levels vary substantially (coefficient of variability: 8% to 27%), mostly due to the effects of diet and of intra- and inter-patient variability in the production, tubular secretion, and renal and extra-renal excretion and degradation of creatinine.
Concurrent use of medications that inhibit tubular secretion or cause haemodynamic changes to renal perfusion
Trimethoprim, cimetidine, and ranitidine can inhibit tubular secretion of creatinine.[66]
Calcineurin inhibitors such as tacrolimus cause dose-dependent vasoconstriction of the afferent arterioles.[67]
Non-steroidal anti-inflammatory drugs inhibit renal prostaglandin production, which reduces renal perfusion.[68]
Artifactual increases
Can occur because of interfering substances or the choice of measurement assay.
Pre-renal causes of elevated creatinine
A reduction in renal perfusion will lead to a decreased GFR, and a resulting rise in levels of serum creatinine. Factors contributing to a reduction in renal perfusion are as follows.
Hypotension
Any cause of hypovolaemic, redistributive, cardiogenic, or obstructive shock can produce a decrease in renal perfusion.
Volume depletion
Causes include diabetic ketoacidosis, gastrointestinal loss, burn injury, and excessive insensible fluid loss.
Renal artery stenosis
Usually due to atherosclerosis of the renal artery, leading to decreased renal perfusion. Consequences include ischaemic nephropathy and renovascular hypertension.
Renal artery thrombosis
A hypercoagulable state or pre-existing renal artery stenosis leads to thrombosis of the renal artery. In patients with normal renal function, the thrombosis usually manifests with a modest increase in serum creatinine and blood pressure. If there is pre-existing renal impairment, renal artery thrombosis can precipitate renal failure, heart failure, and marked hypertension.
Renal vein thrombosis
A hypercoagulable state leads to thrombosis of the renal vein. The most common underlying cause is nephrotic syndrome. Chronic thrombosis is usually covert, but acute thrombosis presents with flank pain and severe haematuria.
Traumatic renal infarction
Traumatic renal infarction occurs in 1% to 2% of all non-penetrating abdominal trauma. Likelihood is increased in the presence of lumbar vertebral injury.[69]
Multiple cholesterol emboli syndrome
Characterised by elevated serum creatinine within days or weeks following arterial manipulation, vascular surgery, stent placement, or cardiac catheterisation. It produces a step-wise acute or subacute progressive rise in serum creatinine, and includes multi-organ involvement (cutaneous lesions, 'thrash toes, blue toes', pancreatitis, stroke, ischaemic bowel, angina).
Congestive heart failure (CHF)
CHF leads to inadequate renal perfusion and an inappropriate activation of the sympathetic and renin-angiotensin systems.
Hepatorenal syndrome
Cirrhosis leads to decreased renal blood flow. The mechanism is poorly understood, but is believed to involve vasoconstriction of the renal circulation as a result of increased portal venous system pressure, suppression of vasodilators, and activation of vasoconstrictors affecting the renal circulation.
Drugs
ACE inhibitors and angiotensin receptor blockers can increase serum creatinine levels by 20% to 30%; the increase is maximised when an ACE inhibitor and angiotensin receptor blocker are used concomitantly.[70] The mechanism is related to a blunted ability of the pre-glomerular circulation to vasodilate following normalisation of blood pressure, leading to hypoperfusion.
Calcineurin inhibitors such as tacrolimus cause dose-dependent vasoconstriction of the afferent arterioles.[67]
Nonsteroidal anti-inflammatory drugs inhibit renal prostaglandin production, which reduces renal perfusion.[68]
Cardiac surgery
Clamping of the main arteries during cardiac surgery leads to diminished renal perfusion, and blood loss during surgery leads to hypovolaemia.
Renal causes of elevated creatinine
Renal causes of elevated creatinine are due to kidney damage, which leads to a decrease in GFR and serum creatinine filtration. Tubulointerstitial diseases also interfere with tubular creatinine secretion.
Primary glomerular disease
Primary glomerular diseases may present with oedema, hypertension, proteinuria, haematuria, elevated creatinine, and other signs and symptoms. A renal biopsy is usually required to diagnose these entities.
Primary glomerular diseases include:
minimal change disease
focal segmental glomerulosclerosis
membranoproliferative (mesangiocapillary) glomerulonephritis
membranous glomerulonephritis
immunoglobulin A (IgA) nephropathy
antiglomerular basement membrane glomerulonephritis (Goodpasture syndrome)
idiopathic crescentic glomerulonephritis
rapidly progressive glomerulonephritis
Secondary glomerular disease
Associated with comorbid conditions such as diabetes mellitus, hypertension, systemic lupus erythematosus, and others. Presenting symptoms may be the same as for primary glomerular diseases.
Diabetic nephropathy: hyperglycaemia triggers inflammation, endothelial dysfunction, and oxidative stress, leading to kidney damage.
Hypertension: systemic hypertension produces high intraglomerular pressure, leading to glomerular damage and a decrease in GFR.
Preeclampsia: produces hypertension and proteinuria. Renal blood flow and GFR are decreased, and creatinine elevation may be seen in the later stages. Elevated serum creatinine during pregnancy may also imply pregnancy-related acute kidney injury or the progression of undetected or known CKD.
Lupus nephritis: renal involvement is present in approximately 50% to 70% of patients with systemic lupus erythematosus.[71] Lupus nephritis is more common in Hispanic and black patients and those with more severe disease in other organ systems. Those with antibodies to double-stranded DNA are more likely to develop glomerulonephritis. Most patients are asymptomatic. Other presentations include hypertension, nephrotic syndrome, or renal failure.
Other vasculitides: a range of vasculitides can produce glomerular damage, leading to a decreased GFR. IgA vasculitis (formerly known as Henoch-Schonlein purpura) is a small-vessel vasculitis affecting the skin, lower extremity, and intestine, and is associated with mesangial IgA depositions in the kidneys. Granulomatosis with polyangiitis is a vasculitic disease affecting the upper respiratory tract, lungs, and kidneys, often leading to fulminant renal failure. Microscopic polyangiitis is a pauci-immune, necrotising, small-vessel vasculitis without necrotising granulomatous inflammation.
Post-infectious glomerulonephritis can be caused by group A beta haemolytic Streptococcus, respiratory and gastrointestinal infections, hepatitis B and C, endocarditis, HIV, toxaemia, syphilis, schistosomiasis, malaria, and leprosy.
Cryoglobulinaemia: cryoglobulins (immunoglobulins that precipitate at cold temperatures) are associated with infectious diseases (hepatitis C), lymphoproliferative disorders (multiple myeloma, Waldenstrom's macroglobulinaemia, chronic lymphocytic leukemia, and B-cell non-Hodgkin's lymphomas) and a range of autoimmune diseases. Kidney damage is produced by immune complex deposition.
Thrombotic microangiopathies: syndromes of microangiopathic haemolytic anaemia, thrombocytopenia, and variable signs of organ impairment due to platelet aggregation in the microcirculation. Kidney damage leads to a decrease in the GFR.
Paraproteinaemias: a group of multi-system disorders characterised by neoplastic proliferation of a single clone of immunoglobulin-producing plasma cells (multiple myeloma, monoclonal gammopathy of undetermined significance, amyloidosis, Waldenstrom's macroglobulinaemia). Protein deposition in the kidney leads to kidney damage, producing a decrease in GFR.
Drugs: penicillamine, sodium aurothiomalate, non-steroidal anti-inflammatory drugs (NSAIDs), captopril, mitomycin C, and ciclosporin can cause glomerulonephritis. Glomerulonephritis may also be caused by the use of heroin.
Hereditary disorders: Fabry's disease, Alport's syndrome, thin basement membrane disease, and nail-patella syndrome can cause glomerulonephritis.
Tubulointerstitial disease
Acute interstitial nephritis: a hypersensitivity reaction usually triggered by an offending medication, that resolves when the triggering medication is stopped. Medications commonly implicated include antibiotics (beta lactams, penicillins, cephalosporins, sulfonamides, rifampicin, quinolones), diuretics, NSAIDs, proton pump inhibitors, cimetidine, ranitidine, allopurinol, phenindione, phenytoin, sulfadiazine, mesalazine, and warfarin. Uncommon causes include sarcoidosis, Sjogren's syndrome, or systemic lupus erythematosus. In some patients, there is no discernible cause.
Acute tubular necrosis: a condition characterised by an abrupt decline in renal function. The pathogenesis is poorly understood. Causes include local or systemic ischaemia from any cause, exogenous nephrotoxins (aminoglycosides, amphotericin-B, poisons [e.g., ethylene glycol], chemotherapeutic agents [e.g., cisplatin], NSAIDs, radiocontrast media, or bacterial toxins), and endogenous nephrotoxins (haem, uric acid, or increased light chain proteins).
Competitive inhibition of creatinine secretion: organic cations tend to compete for common secretory mechanisms. As a result, several drugs are competitive inhibitors of creatinine secretion and increase serum creatinine without affecting GFR. These include cimetidine, gentamicin, fibric acid derivatives other than gemfibrozil, and trimethoprim.
Kidney transplantation: increased serum creatinine following kidney transplantation may indicate transplant rejection, toxicity due to immunosuppressive drugs, or urological complications, and requires urgent assessment.
Physiological adaptation
Kidney donation or unilateral or partial nephrectomy: after an initial rise, serum creatinine decreases due to hyperfiltration by the remaining nephrons and reaches a new steady state. In the long term, serum creatinine remains at 141 to 159 micromol/L (1.6 to 1.8 mg/dL) in patients who have a normal contralateral kidney. Any sustained rise in serum creatinine above the normal range requires further investigation.
Post-renal causes of elevated creatinine
Obstructive uropathy develops when blockage or a narrowing of some part of the urinary tract obstructs urine flow and results in back-pressure on the kidney. This leads to decreased renal blood flow, decreased GFR, and up-regulation of the renin-angiotensin system. This in turn causes atrophy and apoptosis of the renal tubules as well as interstitial fibrosis. The most common causes are:
bladder tumour
benign prostatic hyperplasia
nephrolithiasis
anatomical blockages (e.g. stricture, extrinsic compression of a ureter by a mass in a nearby structure)
iatrogenic ureter damage during surgery, and
retroperitoneal fibrosis.
Use of this content is subject to our disclaimer