History and exam
Key diagnostic factors
common
presence of risk factors
Key risk factors for hypercoagulable state include a history of unprovoked venous thromboembolism (VTE), increasing age, pregnancy/postpartum, malignancy, acute inflammatory state, antiphospholipid antibodies, myeloproliferative disorders, nephrotic syndrome, Behcet's disease, disseminated intravascular coagulation, paroxysmal nocturnal haemoglobinuria, heparin-induced thrombocytopenia, oestrogen-containing oral contraceptive pills/hormone replacement therapy/selective oestrogen receptor modulator therapy, chemotherapy, surgery, antithrombin deficiency, protein C or S deficiency, plasminogen deficiency.
calf swelling
Suggests a deep vein thrombosis. [Figure caption and citation for the preceding image starts]: Acute deep vein thrombosisFrom the collection of Dr Roopen Arya; used with permission [Citation ends].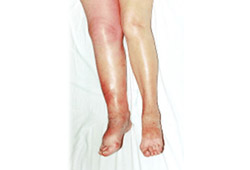
pain or tenderness along deep venous system
Suggests a deep vein thrombosis.
chest pain
Suggests a pulmonary embolism.
tachypnoea
Suggests a pulmonary embolism.
breathlessness
Suggests a pulmonary embolism.
hypotension
Suggests a pulmonary embolism.
tachycardia
Suggests a pulmonary embolism.
Other diagnostic factors
common
family history of venous thromboembolism (VTE)
A history of VTE in first-degree relatives with a first event at a young age (<50 years) may suggest an underlying heritable thrombophilia.[114]
Risk factors
strong
history of unprovoked venous thromboembolism
Approximately 25% of first venous thromboemboli (VTE) are unprovoked (idiopathic) and indicate an underlying hypercoagulable state.[49]
Risk stratification using D-dimer (marker of fibrinolysis), thrombin generation, and factor VIII levels may assist in identifying those at high risk for further VTE.[33][108][109][110][111]
increasing age
Risk of venous thromboembolism increases with age, from 1 in 100,000 children, to 1 in 1000 adults over the age of 40 years, to 1 in 100 in those aged >80 years.[39][40][41] Ageing is associated with increased levels of activated factors VII, IX, and X, and increased levels of factor VIII, fibrinogen, and D-dimer, which are associated with increased risk of thrombosis.[42]
The increased prevalence of comorbidities associated with increasing age can further elevate levels of coagulation factor.
pregnancy/postpartum
Pregnancy results in a physiological fall in protein S, and increases in fibrinogen, factor VIII, and von Willebrand factor. This results in activated protein C resistance. All changes persist for at least 2 months postpartum.[46]
Increases the risk of venous thromboembolism (VTE) fourfold compared with non-pregnant females.[43][44][45]
Although the absolute risk of VTE in pregnancy remains low, it is a leading cause of maternal morbidity and mortality.[47][48] Risk is increased with a family history of VTE and heritable thrombophilia.[7][17][26] Pregnancy-related VTE risk is highest among women with type I antithrombin deficiency, homozygosity for factor V Leiden, or compound heterozygosity,[7][17][26] and those with previous unprovoked oestrogen-associated VTE.
malignancy
Predisposing risk factor in 16% to 18% of patients with thrombophilia.[49][50]
Prothrombotic state results from activation of the coagulation system due to tissue factor expression, fibrinolytic activity, and cytokine release of the malignant cells and their interaction with endothelial cells and platelets.[51][52]
Prevalence of occult malignancy at diagnosis of unprovoked (idiopathic) venous thromboembolism (VTE) is 6.1%, and is 10% at 12 months post VTE diagnosis.[53]
Occult malignancy is less frequent in cases of provoked VTE (1.6% and 2.4% at diagnosis, and 12 months post VTE diagnosis, respectively).[53]
The subsequent diagnosis of cancer is usually advanced metastatic disease.[54]
Occult malignancy and VTE are associated with a high incidence of early recurrence, bleeding, and death.[50]
acute inflammatory state
Risk of venous thromboembolism (VTE) is increased in hospitalised patients with acute infection, arthritis, connective tissue disease, or inflammatory bowel disease.[44][55][56]
Patients in the community with infectious disease have up to a twofold increased risk of VTE.[57]
Inflammatory bowel disease increases the risk of VTE threefold.[58] The underlying mechanism has not been elucidated.
antiphospholipid antibodies (aPLs)
Associated with autoimmune diseases (e.g., systemic lupus erythematosus) or malignancy (e.g., lymphoma).[59]
Most patients with aPLs who develop venous thromboembolism (VTE) have additional risk factors for thrombosis.[59]
Up to 20% of patients presenting with VTE have high levels of anticardiolipin antibodies before the event.[60]
Antiphospholipid syndrome is characterised by persistent aPLs (lupus anticoagulant, anticardiolipin antibodies, anti‐beta-2–glycoprotein 1 antibodies tested at least twice, 12 weeks apart), and either an objectively confirmed thrombotic event or pregnancy-related morbidity.[61][62][63]
Confirmed antiphospholipid syndrome is associated with high risk of recurrent VTE, following withdrawal of anticoagulation.
myeloproliferative disorders
Include polycythaemia vera, essential thrombocythaemia, primary or idiopathic myelofibrosis, and chronic myelogenous leukemia.
Between 12% and 39% of cases present with thrombosis.[64]
High rates of abdominal vein thrombosis are observed.[65]
The JAK2 V617F mutation may be responsible for the prothrombotic phenotype.[65]
nephrotic syndrome
High incidence of hypercoagulable state in first 6 months after diagnosis.[67]
High prevalence of renal vein thrombosis.[68]
Pathogenesis is unclear but probably due to combination of altered levels of coagulation and fibrinolytic proteins, platelet activation, hyperviscosity, hyperlipidaemia, low serum albumin, and therapy with corticosteroids or diuretics.[68]
Behcet's disease
Rare multisystem disorder characterised by recurrent oral and genital ulceration with ocular involvement.
Venous thromboembolism is a common complication, affecting 6% to 39% of patients.[69][70]
The pathogenesis is poorly understood.
Increased markers of thrombin generation and fibrinolysis have been found in all patients.[70]
disseminated intravascular coagulation
Complication of an underlying condition, such as sepsis, malignancy, trauma, or liver disease.
Complex pathogenesis results in increased thrombin generation due to increased tissue factor expression, impaired functioning of natural anticoagulants, and accelerated fibrinolysis.[71]
Can be complicated by bleeding or thrombosis.
paroxysmal nocturnal haemoglobinuria
heparin-induced thrombocytopenia
Caused by formation of antibodies to the complex formed between heparin and platelet factor 4 (PF4).
Antibody binding to heparin/PF4 complex results in platelet activation, microparticle formation, platelet consumption, and thrombocytopenia, and subsequent increased thrombin generation.[74]
Should be suspected in any patient who develops thrombocytopenia or new thrombosis soon after starting heparin therapy.[75][76]
The risk of heparin-induced thrombocytopenia is lowest in medical/obstetric patients treated with low molecular weight heparin (<0.1%) and increases in postoperative cardiac and orthopaedic surgery patients treated with unfractionated heparin (1% to 5%).[75][77]
oestrogen-containing oral contraceptive pill/hormone replacement therapy/selective oestrogen receptor modulator therapy
Increase the risk of venous thromboembolism (VTE).[78][79]
[  ]
]
Absolute risk of VTE with oral contraceptive pill use in young women without personal/family history of venous thrombosis remains low.
Combined oral contraceptive pill and hormone replacement therapy (HRT) can lead to decreased levels of protein S, increased levels of factors VII and VIII, increased fibrinolysis, and activated protein C resistance.[80][81][82][83][84]
Transdermal HRT preparations are associated with a lower risk than oral formulations.[85][86][87][88]
Incidence of VTE is highest in first 12 months of oral contraceptive pill or HRT therapy.[78] Tamoxifen use is associated with an increased risk of VTE.[89] Among women taking adjuvant tamoxifen for early-stage breast cancer, prevalence of factor V Leiden mutation may be approximately five times greater in those who experience a thromboembolic event than in those who do not.[90]
chemotherapy
The overall rate of venous thromboembolism (VTE) for patients receiving chemotherapy for cancer is 6%.
Regimens incorporating thalidomide or lenalidomide, in addition to high-dose dexamethasone, are associated with a higher incidence of VTE (8% to 75% in patients with newly diagnosed myeloma) compared with 3% when thalidomide or lenalidomide is used as a single agent.[91]
Asparaginase therapy results in a near fivefold increase in rate of VTE in acute lymphoblastic leukemia.[92]
surgery
Risk for venous thromboembolism (VTE) varies with type of surgery and other underlying risk factors. In patients not receiving prophylaxis, the baseline risk of symptomatic VTE following major orthopaedic surgery is estimated to be 4.3%.[93]
Possibly due to postoperative prothrombotic state. Surgical trauma may result in tissue factor exposure and thereby activation of coagulation.[94]
antithrombin deficiency
Prevalence is 1 in 5000 in white people.[15]
Genetic mutations cause either a quantitative deficiency or qualitative defect in antithrombin (AT). Reported in up to 3% of white people and 6% of Southeast Asian people with venous thromboembolism (VTE).[10][11][16]
Severe AT deficiency may increase the risk of VTE up to 50-fold.[17]
Homozygosity for an AT defect results in death in utero.
protein C deficiency
Patients who have a well-defined deficiency in protein C are at greater risk of developing venous thromboembolism (VTE) than those who do not.
Prevalence is 1 in 500 in white people, higher in Southeast Asian people.[11][18]
Present in up to 5% of white people and up to 8% of Southeast Asian people with VTE.[10][16][19]
Homozygous deficiency can result in severe phenotype with neonatal purpura fulminans.[20]
protein S deficiency
plasminogen deficiency
Studies suggest that the frequency of plasminogen deficiency is higher than expected in patients with venous thrombosis.[3]
May be a quantitative deficiency (hypoplasminogenaemia) or a qualitative deficiency (dysplasminogenaemia).
weak
obesity
Pathogenesis is multifactorial and includes increased coagulation factors and impaired fibrinolysis.
Tissue factor, factors VII and VIII, and plasminogen activator inhibitor-1 are all increased in obesity and may contribute to the prothrombotic state.[95][96] Adipocytokines may also be involved.[97]
Obesity is associated with increased risk for both first and recurrent venous thromboembolism, with greater risk conferred by increasing body mass index (BMI).[27][98][99]
smoking
HIV infection
The incidence of venous thromboembolism is higher in patients with HIV infection than in age- and sex-matched controls.[103]
The mechanism leading to prothrombotic state is uncertain.
long-haul flight (>4 hours)
Risk of venous thromboembolism (VTE) in a cohort of healthy people taking a flight ≥4 hours is 1 in 6000.[104]
Activation of coagulation and fibrinolysis with increased markers of thrombin generation has been observed in a subset of healthy volunteers after an 8-hour flight. This finding suggests that hypercoagulability acquired in-flight (rather than from immobilisation) contributes to VTE risk.[104][105]
Risk of VTE increases with the presence of additional risk factors.[104][106][107]
elevated fibrinogen
dysfibrinogenaemia
A rare inherited disorder associated with bleeding but complicated by thrombosis in 21% of patients.[21]
factor V Leiden
Genetic mutation of coagulation factor V that confers hypercoagulability.
Prevalence of up to 6% in white people; rare in other ethnic groups.[5][7]
Found in up to 20% of white people with venous thromboembolism (VTE).[22][23] Approximately 90% of patients with activated protein C resistance have factor V Leiden.[3]
Risk of VTE is increased up to sevenfold in heterozygotes and 80-fold in homozygotes.[7]
More than 75% of carriers will never develop VTE.[24] However, among carriers with a family history of VTE, approximately 50% will develop VTE before 65 years of age.[24]
prothrombin gene mutation (G-20210-A; also referred to as F2 c.*97G>A variant)
People carrying the mutation have higher levels of prothrombin than normal.
Prevalence of up to 2% in white people but rare in other ethnic groups.[6][25]
Present in up to 6% of those presenting with venous thromboembolism (VTE) and 18% of those with a positive family history of VTE.[6]
Risk of VTE increased two- to threefold in heterozygotes.
Compound heterozygosity with factor V Leiden increases risk of VTE 20-fold.[26]
elevated factor VIII levels (>150 U/L)
Increases the risk of venous thromboembolism (VTE) up to fivefold, but risk is more than 10-fold in black people.[14][31]
Pathogenesis is unclear; may be due to genetic mutation.[3]
Found in approximately 25% of patients with a VTE.[32]
Risk of VTE increases 10% for every 10 U/litre increment in factor VIII level.[31][32][33]
Associated with increased risk of recurrent VTE.[33]
elevated levels of factor IX or XI
hyperhomocysteinaemia
Role in hypercoagulability is controversial; may not predispose to venous thromboembolism.[37][38]
Can be congenital or acquired. Acquired forms are found in patients with dietary deficiencies of folate, or vitamins B6 or B12. Congenital form is due to a mutation that results in impaired folate binding and reduced methylenetetrahydrafolate reductase (MTHFR) activity.
Potential pathophysiological mechanisms include endothelial activation, proliferation of smooth muscle cells, changes in endothelial nitric oxide production, and changes in endothelial sterol metabolism.[3]
sickle cell disease
Role in producing a hypercoagulable state is controversial.
Sickle cell disease leads to increased levels of markers of thrombin generation, abnormal activation of the fibrinolytic system, increased tissue factor expression, and platelet activation.[27]
It has been suggested that sickle cell disease is associated with increased rates of pulmonary embolism, but deep venous thrombosis (DVT) rates are equivalent to age-matched controls.[28]
elevated levels of thrombin-activatable fibrinolysis inhibitor (TAFI)
Associated with a twofold increase in risk for both first and recurrent venous thromboembolism.[36]
Use of this content is subject to our disclaimer