Aetiology
In normal development, sex development is governed by two consecutive processes: sex determination followed by sex differentiation. Sex determination is the formation of a testis or ovary from a bipotential gonad and is driven by the sequential expression of a number of genes. Sex differentiation is the development of internal and external physical characteristics brought about by gonadal hormone action on target tissues.
[Figure caption and citation for the preceding image starts]: Examples of genes that control different stages of sex determination; ovarian determining genes are the subject of ongoing researchDavies JH, Cheetham T. Recognition and assessment of atypical and ambiguous genitalia in the newborn. Arch Dis Child. 2017;102(10):968-74. [Citation ends].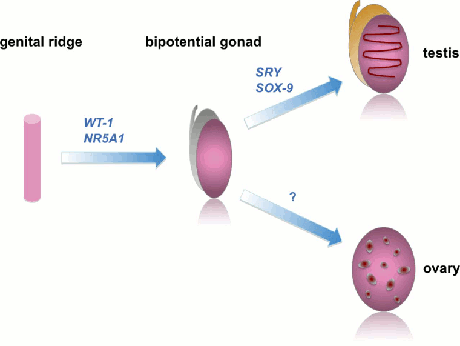 [Figure caption and citation for the preceding image starts]: Schematic representation of sex differentiation. Glossary: 5 alpha-reductase (5α-R), anti-Müllerian hormone (AMH), dihydrotestosterone (DHT)Davies JH, Cheetham T. Recognition and assessment of atypical and ambiguous genitalia in the newborn. Arch Dis Child. 2017;102(10):968-74. [Citation ends].
[Figure caption and citation for the preceding image starts]: Schematic representation of sex differentiation. Glossary: 5 alpha-reductase (5α-R), anti-Müllerian hormone (AMH), dihydrotestosterone (DHT)Davies JH, Cheetham T. Recognition and assessment of atypical and ambiguous genitalia in the newborn. Arch Dis Child. 2017;102(10):968-74. [Citation ends].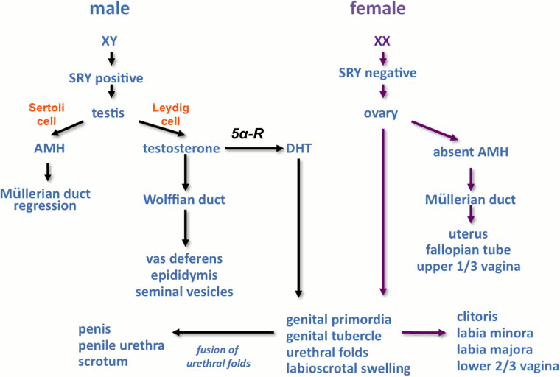 Examples of genes that control different stages of sex determination; ovarian determining genes are the subject of ongoing research.
Examples of genes that control different stages of sex determination; ovarian determining genes are the subject of ongoing research.
Schematic representation of sex differentiation. Glossary: 5 alpha-reductase (5α-R), anti-Müllerian hormone (AMH), dihydrotestosterone (DHT).
A variety of disorders with an underlying genetic basis cause differences of sex development (DSD) and present with atypical genitalia in the neonate. These result from atypical sex determination or sex differentiation. In rare instances, virilisation of a 46,XX fetus may occur due to maternal virilising tumours or maternal exposure to androgenic drugs.
Sex chromosome DSD:
Individuals may present with atypical genitalia due to meiotic or mitotic gain or loss of a sex chromosome, or due to mosaicism (which is rare). 45,X/46,XY mixed gonadal dysgenesis is due to post-meiotic errors, leading to loss of a Y chromosome in one cell lineage. 46,XX/46,XY mosaicism results from the fusion of two zygotes.
46,XY DSD:
Gonadal dysgenesis may be due to mutations in one of several genes that are involved in testicular development.[10] Defects in some of these genes (e.g., sex-determining region Y [SRY]) can also cause ovotesticular DSD.
Disorders of androgen biosynthesis include mutations in genes involved in the pathway of testosterone synthesis that either are common to the adrenal gland and the testes, and thus may also cause deficiencies of glucocorticoids and/or mineralocorticoids (with some forms of congenital adrenal hyperplasia [CAH]), or are just found in the testes and thus only lead to defects in testosterone biosynthesis. Examples are 17 alpha-hydroxylase, 20-22 lyase deficiency due to mutations in CYP17, 3 beta-hydroxysteroid dehydrogenase (HSD) deficiency due to mutations in HSD3B2, and steroidogenic acute regulatory (StAR) protein deficiency. These are all autosomal-recessive conditions.
Partial androgen insensitivity syndrome is due to mutations in the androgen receptor on Xq11, and is an X-linked recessive condition.[11]
Congenital hypogonadotrophic hypogonadism can be due to one of several gene defects and may be isolated or associated with other pituitary hormone deficiencies.[12][13]
46,XX DSD:
Classic CAH due to 21 hydroxylase deficiency (CYP21A2 on chromosome 6p21.3) is the cause of atypical genitalia in more than 95% of cases. It is an autosomal-recessive condition. Other causes of 46,XX DSD include 11 beta-hydroxylase deficiency (CYP11B1 on chromosome 8q21) and 3 beta-HSD deficiency (HSD3B2).
Ovotesticular DSD may be caused by a number of gene defects, including translocation of an SRY gene onto an X chromosome or by duplication of the SOX9 region.[1]
Pathophysiology
An example of a sex chromosome disorder of sex development (DSD) is mixed gonadal dysgenesis, which is characterised by asymmetrical development of the gonads and internal genital ducts. There is a dysgenetic testis and thus poorly developed Wolffian ducts on one side and a streak gonad (ovary) on the other side. Variable degrees of external genital ambiguity are found. The lack of a Y chromosome, and thus lack of the sex-determining region Y (SRY) gene, on one side leads to the dysgenetic testis that produces insufficient testosterone for normal male virilisation. The degree of virilisation depends on the degree of functioning of the testes.
In 46,XY DSD presenting as atypical genitalia, the degree of virilisation depends on the amount of residual functioning of the testes, the amount of testosterone synthesised, or the degree of androgen insensitivity.
In 46,XX DSD with congenital adrenal hyperplasia due to 21 hydroxylase deficiency, the degree of virilisation depends on the residual activity of the 21 hydroxylase gene. In the absence of any 21 hydroxylase activity (null mutations on both alleles), cortisol precursors are shuttled to androgen production leading to elevated androgen levels and virilisation. This is usually associated with salt wasting.[14][Figure caption and citation for the preceding image starts]: Selected examples of abnormal testosterone production (red) or testosterone action (purple) in 46,XY differences of sex development. Glossary: 3 beta-hydroxysteroid dehydrogenase deficiency (3β-HSD), 17 alpha-hydroxylase deficiency (17α-HSD), 17 beta-hydroxysteroid dehydrogenase deficiency (17β-HSD), androgen receptor (AR), complete androgen insensitivity (CAIS), partial androgen insensitivity (PAIS)Davies JH, Cheetham T. Recognition and assessment of atypical and ambiguous genitalia in the newborn. Arch Dis Child. 2017 April [epub ahead of print]. [Citation ends].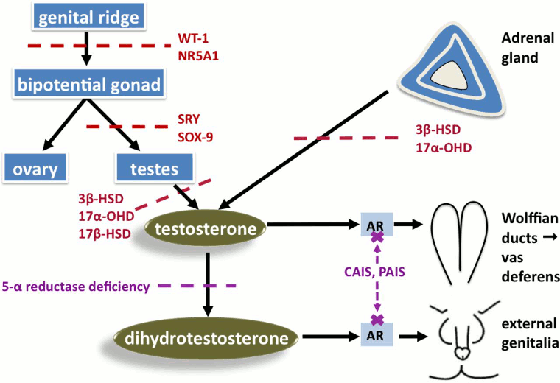 [Figure caption and citation for the preceding image starts]: Selected examples of causes of androgen excess in 46,XX differences of sex developmentDavies JH, Cheetham T. Recognition and assessment of atypical and ambiguous genitalia in the newborn. Arch Dis Child. 2017 April [epub ahead of print]. [Citation ends].
[Figure caption and citation for the preceding image starts]: Selected examples of causes of androgen excess in 46,XX differences of sex developmentDavies JH, Cheetham T. Recognition and assessment of atypical and ambiguous genitalia in the newborn. Arch Dis Child. 2017 April [epub ahead of print]. [Citation ends].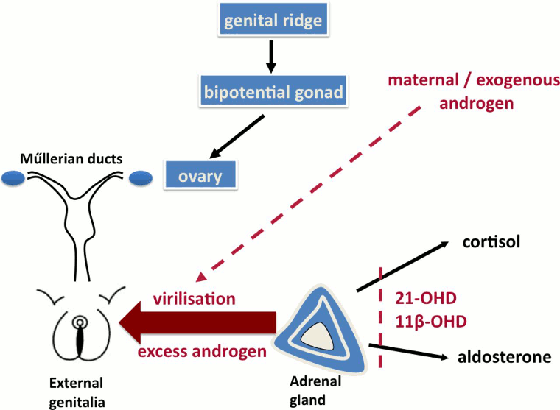
Use of this content is subject to our disclaimer